Question: 1. A liquid phase reaction has kinetics as given below. In order to achieve BC2 what should be the type and order of two consecutive
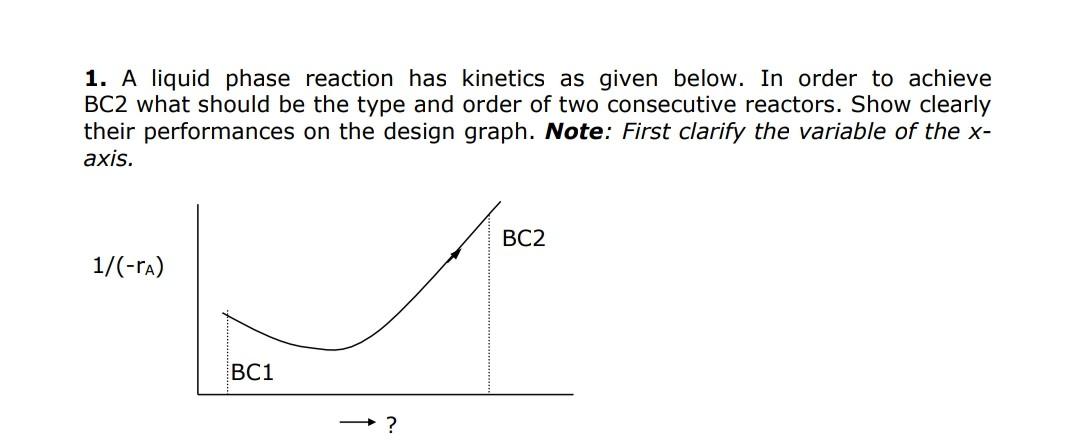
1. A liquid phase reaction has kinetics as given below. In order to achieve BC2 what should be the type and order of two consecutive reactors. Show clearly their performances on the design graph. Note: First clarify the variable of the x axis. 1. A liquid phase reaction has kinetics as given below. In order to achieve BC2 what should be the type and order of two consecutive reactors. Show clearly their performances on the design graph. Note: First clarify the variable of the x axis
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


