Question: 1) at equilibrium, the forward and reverse reaction rate constants are equal 2) when Q equals 1, the forward and reverse reaction rates are always
1) at equilibrium, the forward and reverse reaction rate constants are equal
2) when Q equals 1, the forward and reverse reaction rates are always equal
3) when Q=K, the forward and reverse reaction rates are always equal
4) when Q>K, the reverse reaction rate will be greater than the forward reaction rate
5) at equilibrium, the concentration of A is twice that of B
6) none of the statements are true 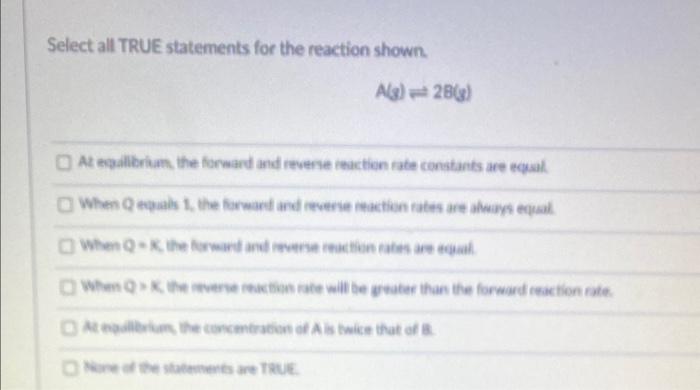

Select all TRUE statements for the reaction shown. A(3)2B(3) At eqailbriam. the forward and revere ieactien rate conatants are equal. When Q equabs 1. the forward and iveerse inaction rates are atwieys equal At noalleriums, the coscmeraticit of A is twice that of A. Wire of the statomerts are RRUE
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


