Question: Hi, I don't get why when a system cools, q has positive sign(endothermic) and when the system heats up, q has negative sign(exothermic). Shouldn't it
Hi, I don't get why when a system cools, q has positive sign(endothermic) and when the system heats up, q has negative sign(exothermic).
Shouldn't it be the opposite?
endothermic is when you recieve heat, so doesn't is indicate heating up?
thanks!
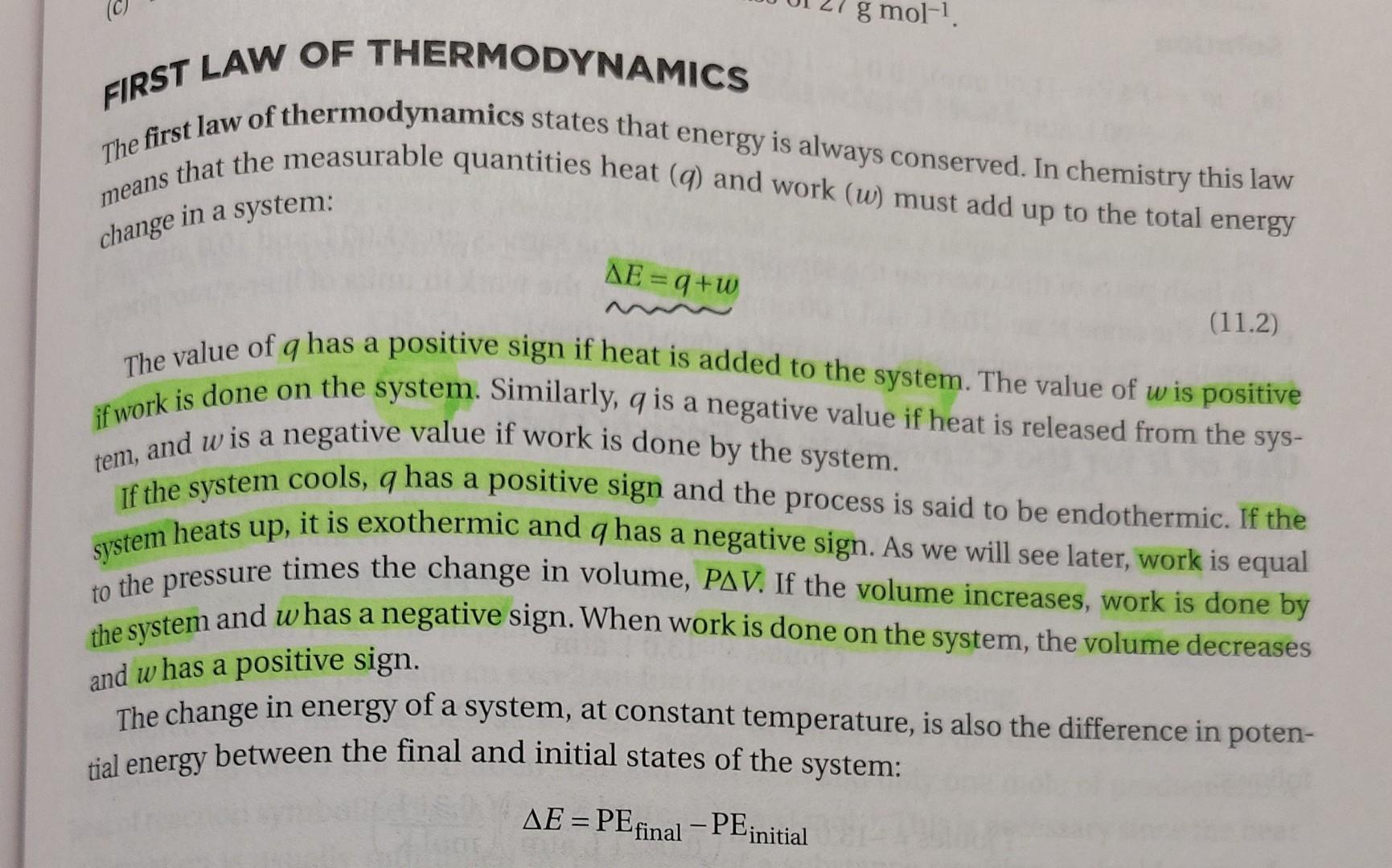
FIRST LAW OF THERMODYNAMICS The first law of thermodynamics states that energy is always conserved. In chemistry this law me eans that the measurable quantities heat (q) and work (w) must add up to the total energy change in a system: E=q+w The value of q has a positive sign if heat is added to the system. The value of w is positive if work is done on the system. Similarly, q is a negative value if heat is released from the system, and w is a negative value if work is done by the system. If the system cools, q has a positive sign and the process is said to be endothermic. If the system heats up, it is exothermic and q has a negative sign. As we will see later, work is equal to the pressure times the change in volume, PV. If the volume increases, work is done by the system and w has a negative sign. When work is done on the system, the volume decreases and w has a positive sign. The change in energy of a system, at constant temperature, is also the difference in potential energy between the final and initial states of the system: E=PEfinalPEinitial
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


