Question: 1 - Atomic radius. crystal structure, electronegativity, and the most common valence are tabutatud in the following table for several elements for those that are
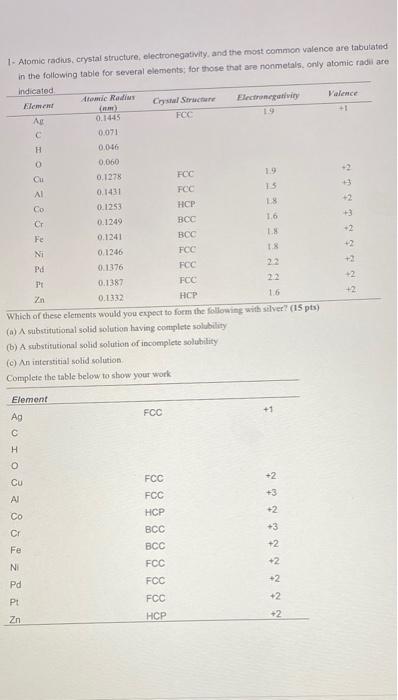
1 - Atomic radius. crystal structure, electronegativity, and the most common valence are tabutatud in the following table for several elements for those that are nonmetals, only atomic radi are indicated teme Radio Crystal Structure Hlement Valence Electronegativity (A) 19 All FCC 0.1445 c. 0.071 H 0.006 0 0.060 Cu -2 FCC 0.1278 1.9 Al 3 FCC 0.1431 13 Co 0.1253 HCP 42 18 Cr 0.1249 Fe 0.1241 BCC NI 0.1246 FCC 1.8 Pd 0.1376 FCC PE 0.1387 FCC 22 2n 0.1332 HCP 16 Which of these elements would you expect to form the following with silver? (15 pts) (a) A substitutional solid solution having complete solubility (b) A substitutional solid solution of incomplete solubility (c) An interstitial solid solution Complete the table below to show your work 16 Element Ag FCC +1 +2 Cu +3 +2 u 2 IO 3 3 8 25 -3 FCC FCC HCP BCC BCC FCC FCC FCC +2 +2 +2 Pd +2 Zn +2 HCP
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


