Question: 2.2 Below, atomic radius, crystal structure, electronegativity, and the most common valence are tabulated, for several elements; for those that are nonmetals, only atomic radii
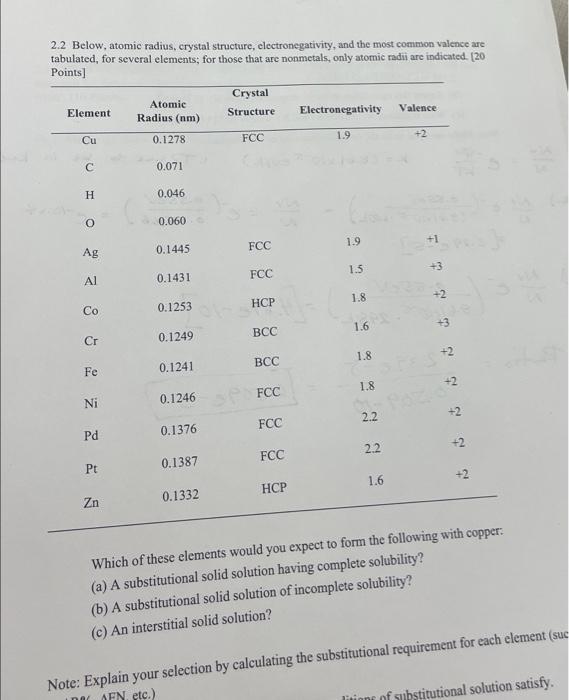
2.2 Below, atomic radius, crystal structure, electronegativity, and the most common valence are tabulated. for several elements for those that are nonmetals, only atomic radil are indicated. [20 Which of these elements would you expect to form the following with copper: (a) A substitutional solid solution having complete solubility? (b) A substitutional solid solution of incomplete solubility? (c) An interstitial solid solution? Note: Explain your selection by calculating the substitutional requirement for each element (su
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


