Question: 1 b) An aqueous solution containing a valuable solute is colored by small amounts of an impuri is to be removed by adsorption. The equilibrium
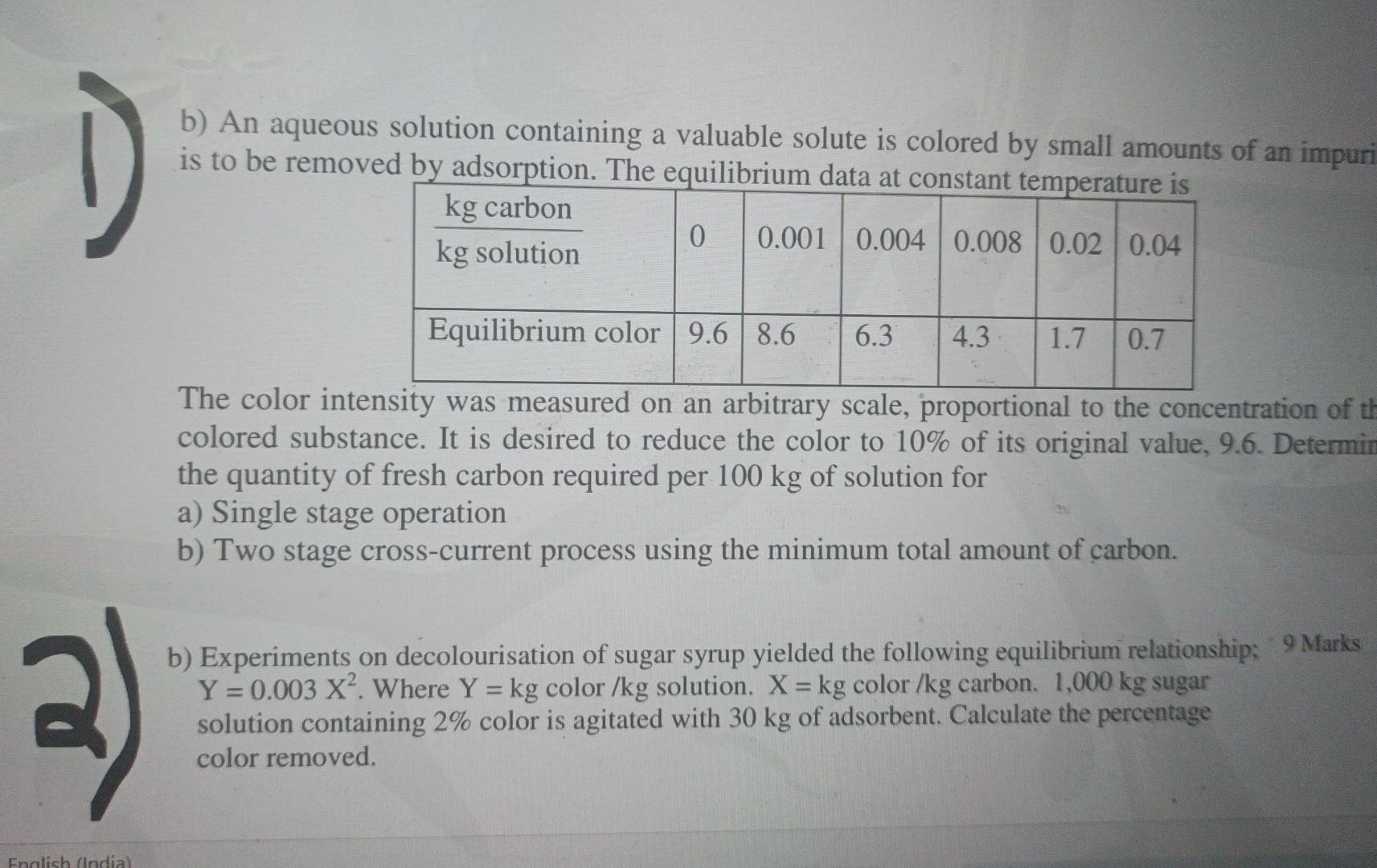
1 b) An aqueous solution containing a valuable solute is colored by small amounts of an impuri is to be removed by adsorption. The equilibrium data at constant temperature is kg carbon 0 0.001 0.004 0.008 0.02 0.04 kg solution Equilibrium color 9.6 8.6 6.3 4.3 1.7 0.7 The color intensity was measured on an arbitrary scale, proportional to the concentration of th colored substance. It is desired to reduce the color to 10% of its original value, 9.6. Determin the quantity of fresh carbon required per 100 kg of solution for a) Single stage operation b) Two stage cross-current process using the minimum total amount of carbon. -- b) Experiments on decolourisation of sugar syrup yielded the following equilibrium relationship: 9 Marks Y = 0.003 X?. Where Y = kg color /kg solution. X = kg color/kg carbon. 1,000 kg sugar solution containing 2% color is agitated with 30 kg of adsorbent. Calculate the percentage color removed. English (Indial
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


