Question: 1. Convert the molecules below in to the most stable and least stable chair conformations and circle the most stable one. H3C HC. CH, Br
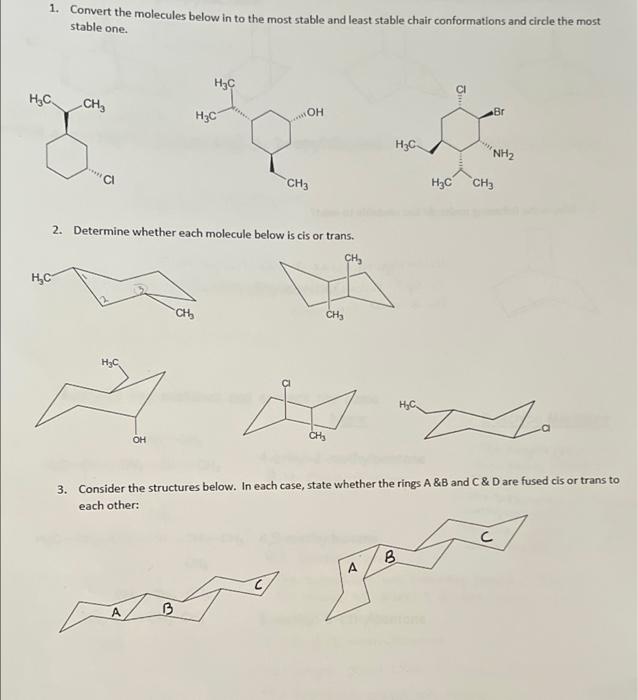
1. Convert the molecules below in to the most stable and least stable chair conformations and circle the most stable one. H3C HC. CH, Br HC "NH2 CH3 H3C CH3 2. Determine whether each molecule below is cis or trans. CH, HC "CH, CH T CH 3. Consider the structures below. In each case, state whether the rings A&B and C&D are fused cis or trans to each other: B A B
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


