Question: 1. Decide whether the compounds shown below are ionic compounds or covalent compounds and give their systematic names. For the ionic compounds, specify the charge
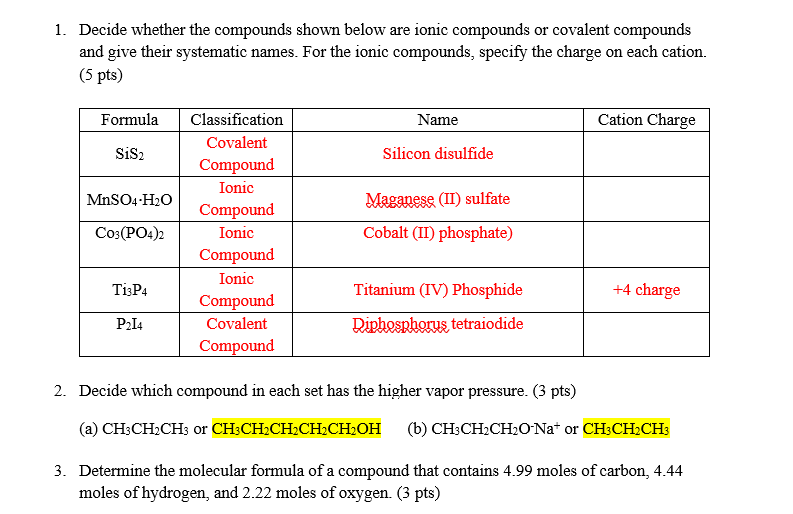
1. Decide whether the compounds shown below are ionic compounds or covalent compounds and give their systematic names. For the ionic compounds, specify the charge on each cation. (5pts) 2. Decide which compound in each set has the higher vapor pressure. ( 3pts) (a) CH3CH2CH3 or CH3CH2CH2CH2CH2OH (b) CH3CH2CH2ONa+or 3. Determine the molecular formula of a compound that contains 4.99 moles of carbon, 4.44 moles of hydrogen, and 2.22 moles of oxygen. ( 3 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


