Question: For the following process, determine the change in entropy for the system (AS sys), the surrounding (A.S sys), and the universe (AS univ). Then
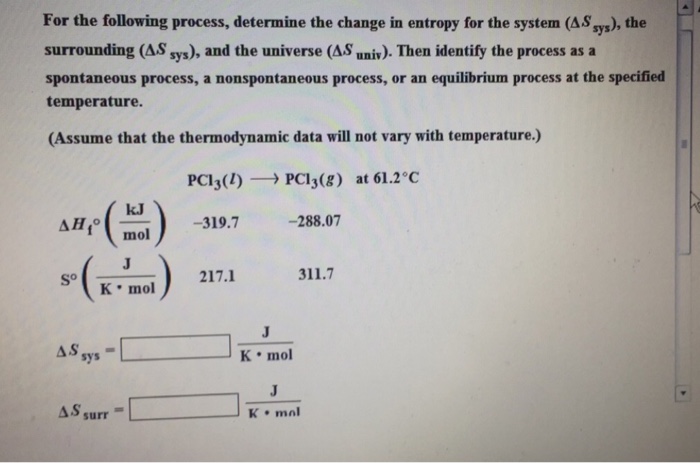
For the following process, determine the change in entropy for the system (AS sys), the surrounding (A.S sys), and the universe (AS univ). Then identify the process as a spontaneous process, a nonspontaneous process, or an equilibrium process at the specified temperature. (Assume that the thermodynamic data will not vary with temperature.) PC13(1) PC13(g) at 61.2C So J K mol AS sys kJ mol AS surr -319.7 217.1 -288.07 J K mol . 311.7 J K mal
Step by Step Solution
3.51 Rating (175 Votes )
There are 3 Steps involved in it
The image youve provided contains a question regarding thermodynamics Specifically its asking to calculate the change in entropy for a system Ssys sur... View full answer

Get step-by-step solutions from verified subject matter experts


