Question: 1; Determine for which catalyst particle radius, the effectiveness factor is equal to 0.8 2; For this particle size, calculate the catalyst mass required for
1; Determine for which catalyst particle radius, the effectiveness factor is equal to 0.8 2; For this particle size, calculate the catalyst mass required for 80 % conversion.
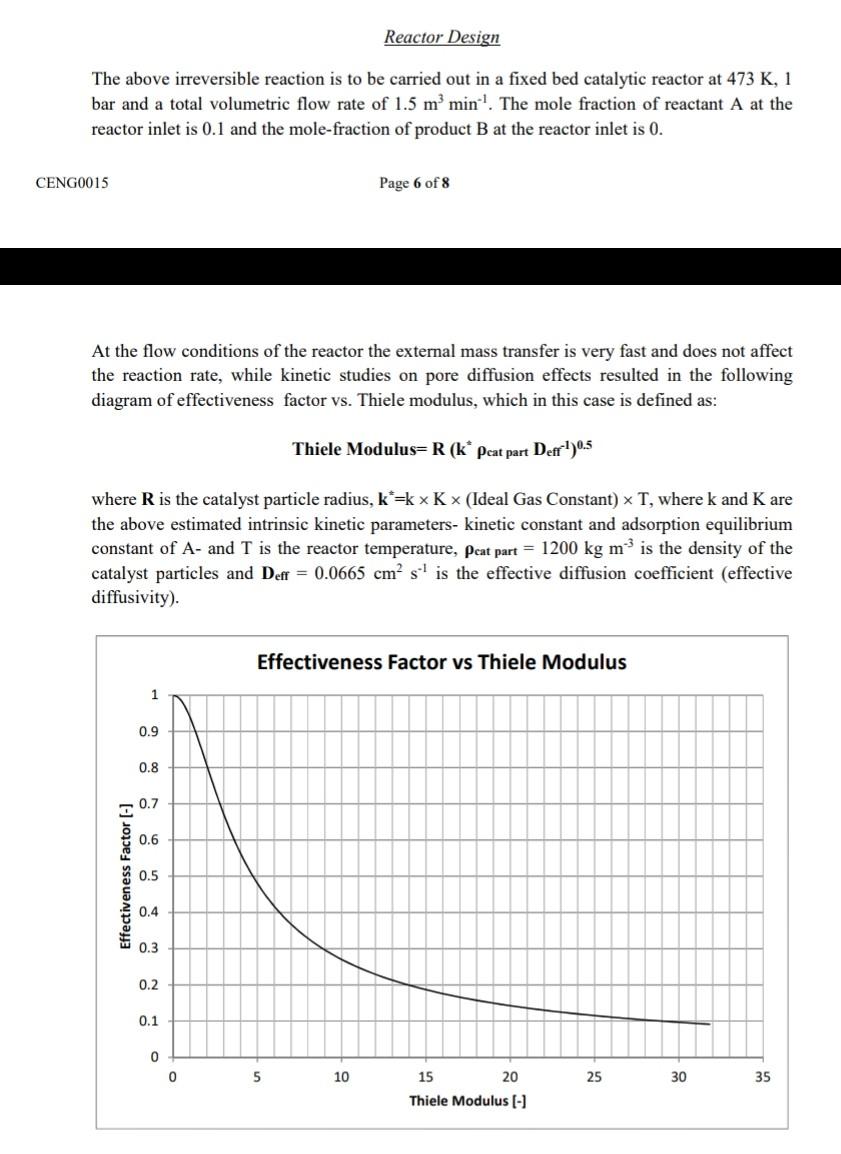
Reactor Design The above irreversible reaction is to be carried out in a fixed bed catalytic reactor at 473 K, 1 bar and a total volumetric flow rate of 1.5 m min. The mole fraction of reactant A at the reactor inlet is 0.1 and the mole-fraction of product B at the reactor inlet is 0. CENG0015 Page 6 of 8 At the flow conditions of the reactor the external mass transfer is very fast and does not affect the reaction rate, while kinetic studies on pore diffusion effects resulted in the following diagram of effectiveness factor vs. Thiele modulus, which in this case is defined as: Thiele Modulus=R (k* peat part Defr!)0.5 where R is the catalyst particle radius, k*rk x K x (Ideal Gas Constant) x T, where k and K are the above estimated intrinsic kinetic parameters- kinetic constant and adsorption equilibrium constant of A- and I is the reactor temperature, peat part = 1200 kg m is the density of the catalyst particles and Deff = 0.0665 cm s' is the effective diffusion coefficient (effective diffusivity). Effectiveness Factor vs Thiele Modulus 1 0.9 0.8 0.7 0.6 Effectiveness Factor (-) 0.5 0.4 0.3 0.2 0.1 0 0 5 10 25 30 35 15 20 Thiele Modulus (-) Reactor Design The above irreversible reaction is to be carried out in a fixed bed catalytic reactor at 473 K, 1 bar and a total volumetric flow rate of 1.5 m min. The mole fraction of reactant A at the reactor inlet is 0.1 and the mole-fraction of product B at the reactor inlet is 0. CENG0015 Page 6 of 8 At the flow conditions of the reactor the external mass transfer is very fast and does not affect the reaction rate, while kinetic studies on pore diffusion effects resulted in the following diagram of effectiveness factor vs. Thiele modulus, which in this case is defined as: Thiele Modulus=R (k* peat part Defr!)0.5 where R is the catalyst particle radius, k*rk x K x (Ideal Gas Constant) x T, where k and K are the above estimated intrinsic kinetic parameters- kinetic constant and adsorption equilibrium constant of A- and I is the reactor temperature, peat part = 1200 kg m is the density of the catalyst particles and Deff = 0.0665 cm s' is the effective diffusion coefficient (effective diffusivity). Effectiveness Factor vs Thiele Modulus 1 0.9 0.8 0.7 0.6 Effectiveness Factor (-) 0.5 0.4 0.3 0.2 0.1 0 0 5 10 25 30 35 15 20 Thiele Modulus (-)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


