Question: 1. Exercise 4.26 Sodium hydroxide, NaOH, may be prepared by reacting calcium hydroxide, Ca(OH)2, with sodium carbonate, Na2CO3. Ca(OH)2(aq)+Na2CO3(aq)2NaOH(aq)+CaCO3(s) How many milliliters of 0.150MCa(OH)2 will
1.
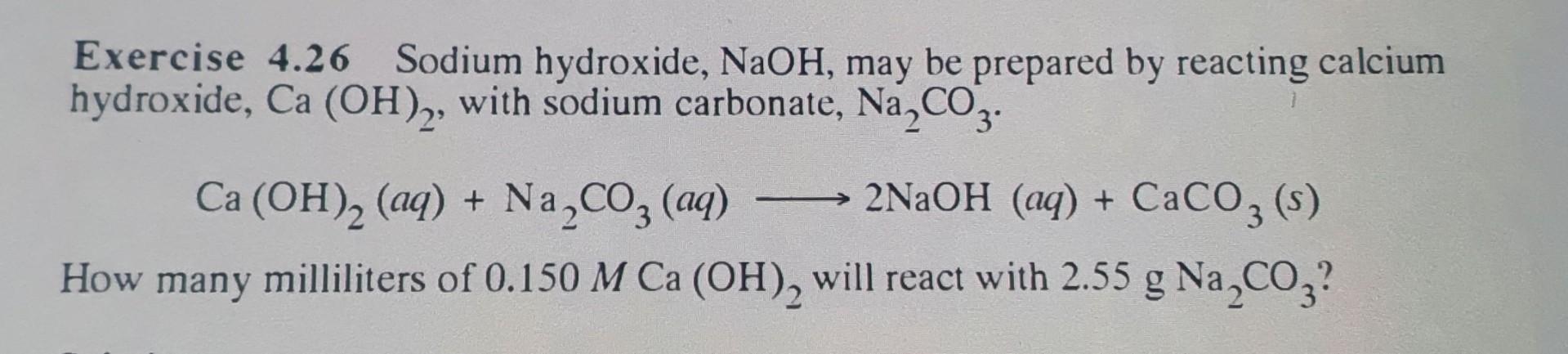
Exercise 4.26 Sodium hydroxide, NaOH, may be prepared by reacting calcium hydroxide, Ca(OH)2, with sodium carbonate, Na2CO3. Ca(OH)2(aq)+Na2CO3(aq)2NaOH(aq)+CaCO3(s) How many milliliters of 0.150MCa(OH)2 will react with 2.55gNa2CO3
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


