Question: 1. First evaluate the reactants side: Start with CH H 2 +1 +2 CH number of atoms oxidation state total charge C 2 -1
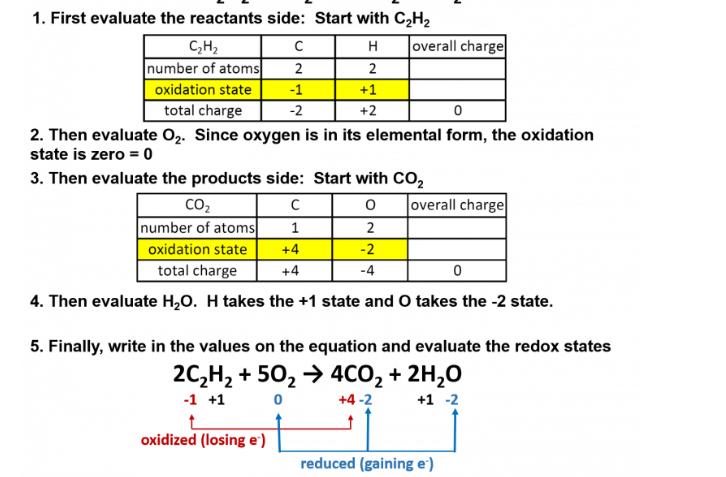
1. First evaluate the reactants side: Start with CH H 2 +1 +2 CH number of atoms oxidation state total charge C 2 -1 -2 overall charge 0 2. Then evaluate O. Since oxygen is in its elemental form, the oxidation state is zero = 0 3. Then evaluate the products side: Start with CO 0 1 2 +4 -2 +4 -4 CO number of atoms oxidation state total charge 0 4. Then evaluate HO. H takes the +1 state and O takes the -2 state. oxidized (losing e') overall charge 5. Finally, write in the values on the equation and evaluate the redox states 2CH +50 4CO + 2HO -1 +1 0 +4-2 +1 -2 reduced (gaining e')
Step by Step Solution
There are 3 Steps involved in it
Answer The given chemical equation is 2CH 5O 4CO 2HO To determine which substance is oxidized and wh... View full answer

Get step-by-step solutions from verified subject matter experts


