Question: 1. For each problem below, circle the best answer. In each case, there is only one correct answer A. Which alkene would be most reactive
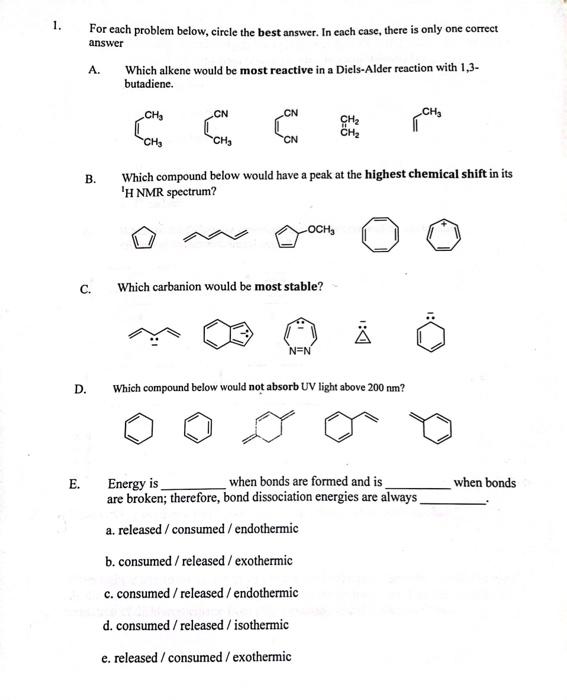

1. For each problem below, circle the best answer. In each case, there is only one correct answer A. Which alkene would be most reactive in a Diels-Alder reaction with 1,3butadiene. B. Which compound below would have a peak at the highest chemical shift in its 'H NMR spectrum? C. Which carbanion would be most stable? D. Which compound below would not absorb UV light above 200nm ? E. Energy is when bonds are formed and is when bonds are broken; therefore, bond dissociation energies are always a. released / consumed / endothermic b. consumed / released / exothermic c. consumed / released/endothermic d. consumed / released / isothermic e. released/consumed/exothermic F. Consider the bond dissociation energies listed below in kcal/mol. CH3Br=70CH3CH2Br=68(CH3)2CHBr=68(CH3)3CBr=65 These data show that the CBr bond is weakest when bromine is bound to a a. methyl carbon b. quatemary carbon c. primary carbon d. 30 carbon e. 2 carbon Which of the following represents the energy levels of the molecular orbitals of cyclopentadienyl anion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


