Question: 1) Given the reactions (a) and (b) below, determine rH and rU for reaction (c), all at 298K. Assume ideal gas law is valid for
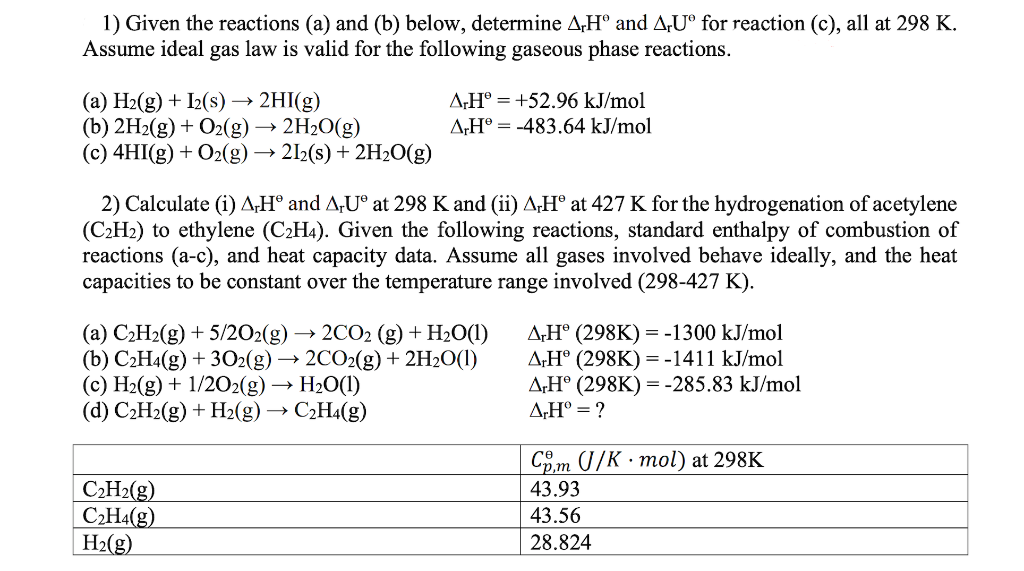
1) Given the reactions (a) and (b) below, determine rH and rU for reaction (c), all at 298K. Assume ideal gas law is valid for the following gaseous phase reactions. (a) H2(g)+I2(s)2HI(g)rHe=+52.96kJ/mol (b) 2H2(g)+O2(g)2H2O(g)rH=483.64kJ/mol (c) 4HI(g)+O2(g)2I2(s)+2H2O(g) 2) Calculate (i) rH and rU at 298K and (ii) rH at 427K for the hydrogenation of acetylene (C2H2) to ethylene (C2H4). Given the following reactions, standard enthalpy of combustion of reactions (a-c), and heat capacity data. Assume all gases involved behave ideally, and the heat capacities to be constant over the temperature range involved (298-427 K)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


