Question: 1. List all intermolecular interactions that occur between the following molecules. 2. Identify the strongest intermolecular force present in a pure liquid sample of each
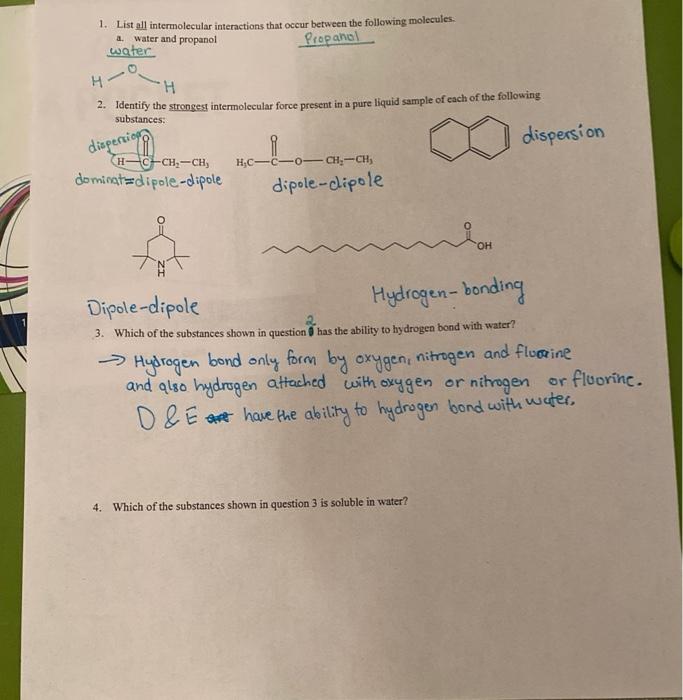
1. List all intermolecular interactions that occur between the following molecules. 2. Identify the strongest intermolecular force present in a pure liquid sample of each of the following: substances: dispersion dominat = dipole-dipole dipole-dipole Dipole-dipole Hydrogen-bonding 3. Which of the substances shown in question has the ability to hydrogen bond with water? Hydrogen bond only form by oxygen, nitrogen and fluorine and also hydrogen attached with oxygen or nitrogen or fluorine. D E have the ability to hydrogen bond with water. 4. Which of the substances shown in question 3 is soluble in water
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


