Question: 1. Note the average atomic mass of the elements on the nano-balance. Add atoms to the larger balance until it registers the same number in
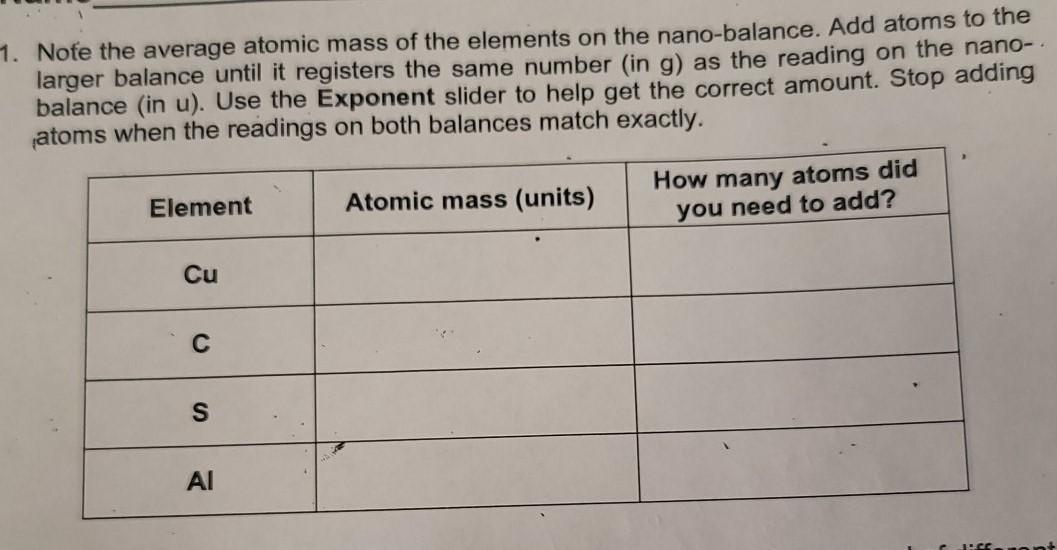
1. Note the average atomic mass of the elements on the nano-balance. Add atoms to the larger balance until it registers the same number in g) as the reading on the nano- balance (in u). Use the Exponent slider to help get the correct amount. Stop adding atoms when the readings on both balances match exactly. Element Atomic mass (units) How many atoms did you need to add? Cu C S
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


