Question: 1. One resonance form (structure) tor CH4N2O is shown. a. Draw the two other resonance contributors in the boxes, include all non-bonding electrons and formal
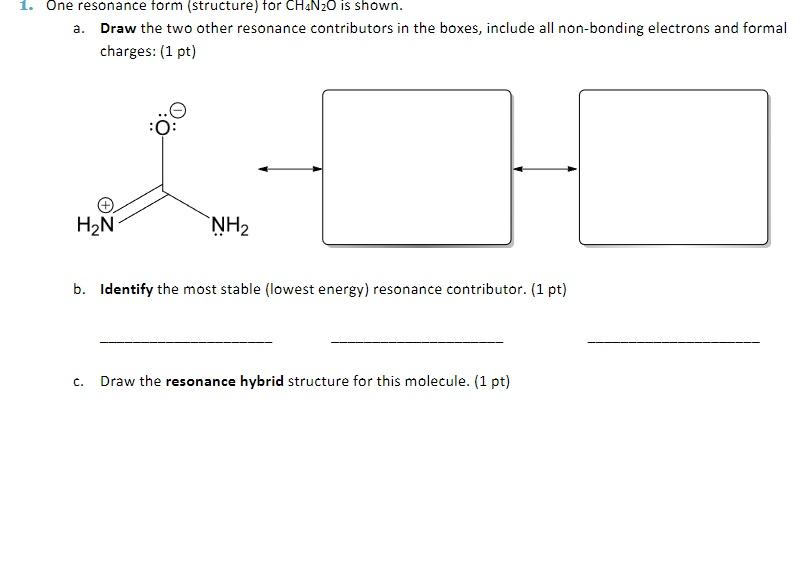
1. One resonance form (structure) tor CH4N2O is shown. a. Draw the two other resonance contributors in the boxes, include all non-bonding electrons and formal charges: (1 pt) b. Identify the most stable (lowest energy) resonance contributor. ( 1pt ) c. Draw the resonance hybrid structure for this molecule. (1 pt)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


