Question: 1. Place 0.5 g of well-powdered 3-nitrobenzoic acid and a stirring bar into a 10 mL round bottom flask. (The powdering is best done




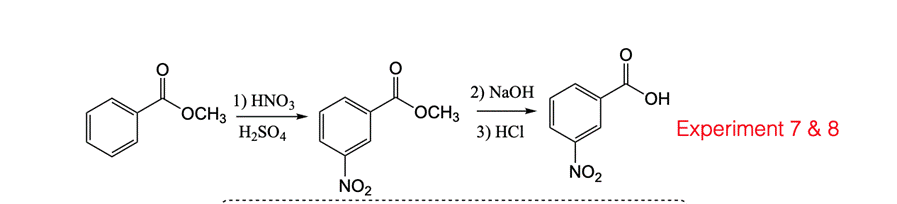
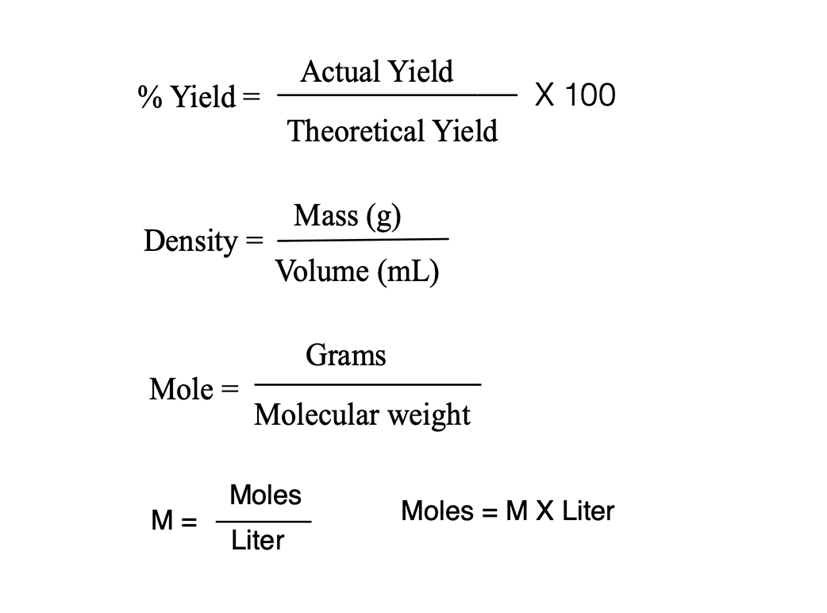
1. Place 0.5 g of well-powdered 3-nitrobenzoic acid and a stirring bar into a 10 mL round bottom flask. (The powdering is best done with a 25 mL Erlenmeyer and smooth surfaced weighing paper.) 2. Attach a drying tube containing a wad of cotton and a one-inch layer of calcium chloride. 3. Under a fume hood, clamp the assembly in a water bath, making sure the assembly is half submerged in the water, and a thermoprobe is clamped in the water as well. 4. Remove the flask assembly and set it aside for the moment. Adjust the hot plate to warm the water bath to 50C. 5. Take the flask assembly to the chemical supply hood and temporarily remove the drying tube assembly and add 0.4 mL of thionyl chloride (d = 1.63 g/mL) using the attached 1.0 mL cali- brated pipette. Then add 6 drops of dimethylformamide (DMF) to the flask and replace the drying tube. Do this entire step in the chemical supply hood only, do not remove the thionyl chloride bottle or its associated pipette from that hood. 6. Bring the flask back to the hood with your 50C water bath, clamp the assembly in place and stir the mixture for 10 minutes at 50C. It is essential to keep the temperature of the water bath at 50 t 5C. Do not start tining if the temperature is below 45C. 7. After the flask assembly is warming in the water bath place a 50 mL Erlenmeyer flask contain- ing 12 mL of concentrated ammonia (15 M) in an ice bath in the same hood, close to your water bath. Note: In research labs, often such water-sensitive reactions are performed under an inert atmosphere (argon or nitrogen gas) rather than using a drying tube. 8. If the entire sample has not liquified after approximately 10 minutes, remove the flask assembly from the water bath, take it to the chemical supply hood and add another 0.2 mL of thionyl chloride to the mixture, as before. 9. Bring the flask assembly back to your water bath and continue heating until no solid remains. Continuous stirring also helps to complete the reaction. 10. Once the reaction is complete, raise the reaction flask above the water bath and remove the drying tube. Use an eyedropper to transfer the acid chloride solution dropwise (slowly, at a rate of 1 drop per two seconds) to the ice-cold flask of ammonia while stirring. Keep the ammonia solution in the ice bath during the addition of 3-nitrobenzoyl chloride. REMEMBER TO KEEP YOUR HEAD OUT OF THE EXHAUST HOODS AT ALL TIMES DURING THIS EXPERIMENT. BOTH HCI AND SO, ARE EXTREMELY POISONOUS GASES. 11. Maximum yields are obtained when the addition is performed with cold ammonia, which is stirred continuously. Keeping 1-2 pieces of ice in the ammonia solution during the addition will help keep the temperature close to 0C. Inverting the eyedropper allows the acyl chloride to enter the rubber bulb, contaminating your product and destroying the rubber. Do not hold an eyedropper upside down. 12. When the addition is complete, rinse the round bottom flask and the eyedropper with some ammonia. The materials may now be safely removed from the hood if the ammonia flask is stoppered. Otherwise, cool it in the hood. 13. Cool the crude amide for a few minutes, and collect the solid by suction filtration on a Hirsch funnel. A 1.5 cm piece of filter paper should just cover the bottom of the funnel, the same as a Bchner funnel. 14. Wash the product well with water to remove a large amount of NH,CI. Pour this ammonia waste down the drain promptly, and rinse with water. 15. Use a 10 mL pear-shaped flask to recrystallize the crude amide by dissolving it in a mini- mum amount of denatured ethanol with heating (1-2 mL depending on the yield of your 3-nitrobenzamide). 16. Then add enough water to just saturate (first cloudiness) the solution followed by addition of 1-2 drops of ethanol. Do not add more than 5 mL of water in any case, as the product is also somewhat soluble in water. 17. The above crystallization is a difficult one to perform successfully. It is always a good idea to keep a few crystals of the crude product for "seeding" in case you have trouble with the recrys- tallization. Remember, also, that a trace of ammonium chloride may be trapped in the crude amide it will be virtually insoluble in denatured ethanol so you may have to filter it. 18. Use your Hirsch funnel to collect the product by suction filtration after cooling the 10 mL pear-shaped flask in an ice bath for five minutes. The mother liquor should be added to the Hazardous Waste Container. 19. If you have not already done so, return the calcium chloride to the solid waste container. Calcium chloride left in drying tubes will eventually form a solid cake that is almost impossible to remove. 20. Simultaneously determine the melting point of the starting 3-nitrobenzoic acid, the final amide, and an intimately ground mixture of the two solids. The amide and corresponding acid both melt near 130C. The range is from the first appearance of liquid until the complete disappear- ance of solid. It should be quite wide for a mixed melting point. Weight of 3-nitrobenzamide = 0.28 g m.p. of 3-nitrobenzamide = 137.5 C 1) O 2) NaOH OH OCH3 OCH3 H2SO4 3) HCI Experiment 7 & 8 NO2 NO2 Actual Yield % Yield X 100 Theoretical Yield Mass (g) Density %| Volume (mL) Grams Mole = %3D Molecular weight Moles M = Moles = M X Liter Liter
Step by Step Solution
3.61 Rating (155 Votes )
There are 3 Steps involved in it
Based on the provided instructions here are the steps for the chemical reaction setup 1 Take a 10 mL round bottom flask and add 05 g of wellpowdered 3nitrobenzoic acid and a stirring bar 2 Attach a dr... View full answer

Get step-by-step solutions from verified subject matter experts


