Question: 1 point A metal crystalizes in a face-centered cubic unit cell, The radius of the atom is 1441010cm. The density of the element is 19.32gcm3.
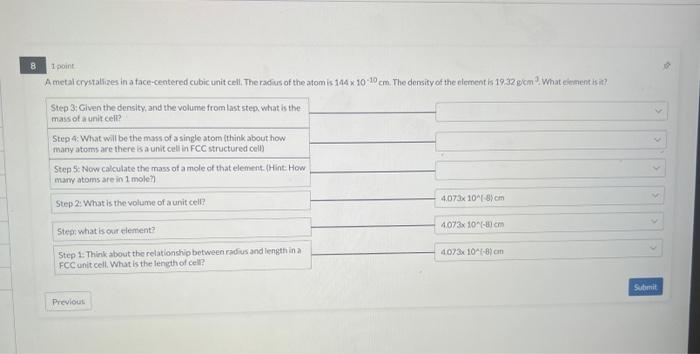
1 point A metal crystalizes in a face-centered cubic unit cell, The radius of the atom is 1441010cm. The density of the element is 19.32gcm3. What eirnent is in
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


