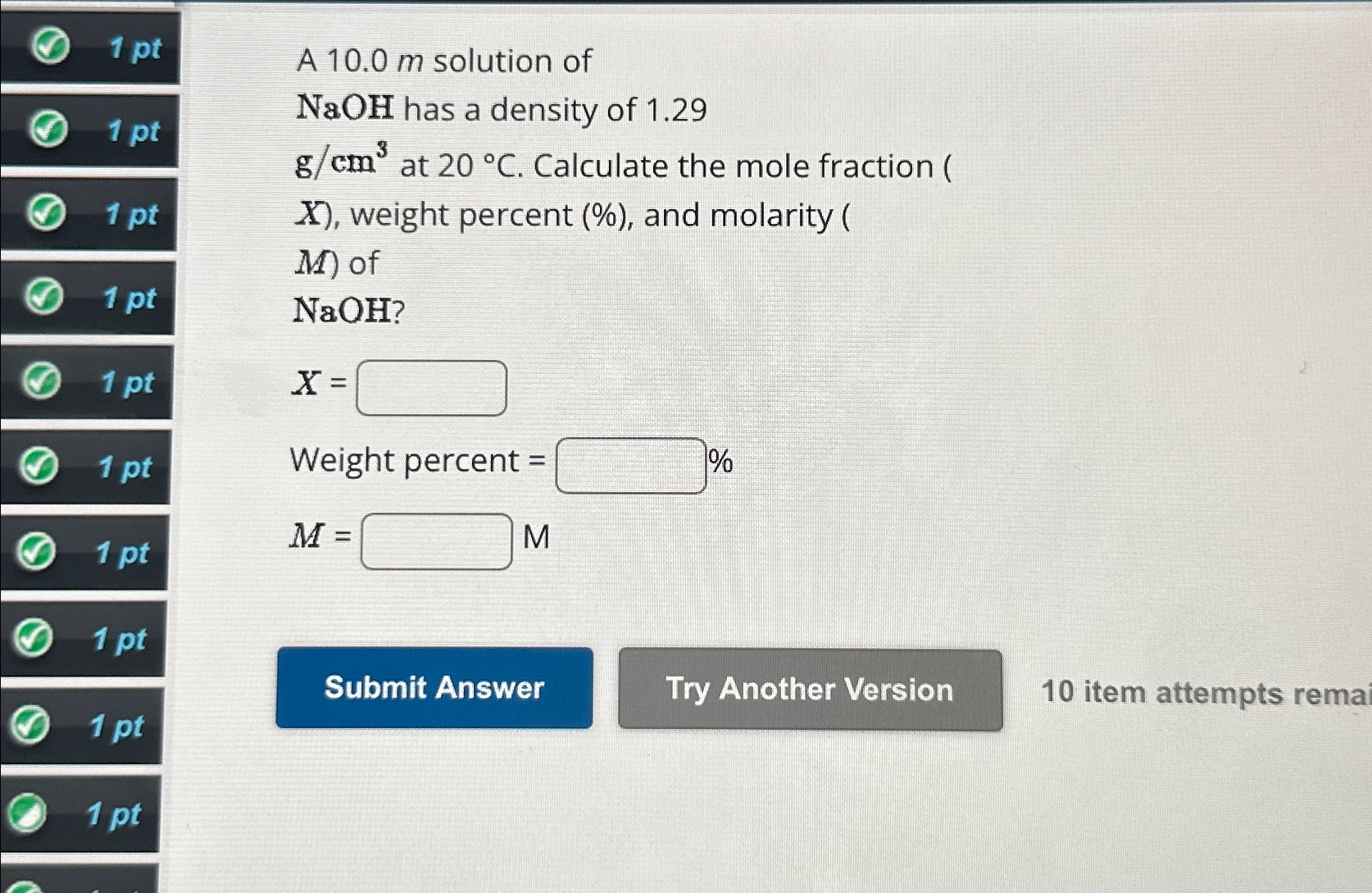
Question: 1 pt 1pt 1pt 1 pt 1pt 1pt 1 pt 1 pt 1 pt 1 pt A 10.0m solution of NaOH
1 pt\
1pt\
1pt\ 1 pt\
1pt\
1pt\ 1 pt\ 1 pt\ 1 pt\ 1 pt\ A
10.0msolution of\
NaOHhas a density of 1.29\
(g)/(c)m^(3)at
20\\\\deg C. Calculate the mole fraction (\
x, weight percent (%), and molarity (\
M) of\
NaOH?\
x=\ Weight percent
=\
%\
M=,M\ 10 item attempts rema
A 10.0m solution of NaOH has a density of 1.29 g/cm3 at 20C. Calculate the mole fraction ( X ), weight percent (\%), and molarity ( M ) of NaOH ? X= Weight percent =% M=M 10 item attempts rema A 10.0m solution of NaOH has a density of 1.29 g/cm3 at 20C. Calculate the mole fraction ( X ), weight percent (\%), and molarity ( M ) of NaOH ? X= Weight percent =% M=M 10 item attempts rema
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


