Question: - 1 The feed to a batch combustion chamber consists of 3.25 moles of methanol and 9.75 moles of Oz. CH4O+A02BCO2+ CH20 Determine the values
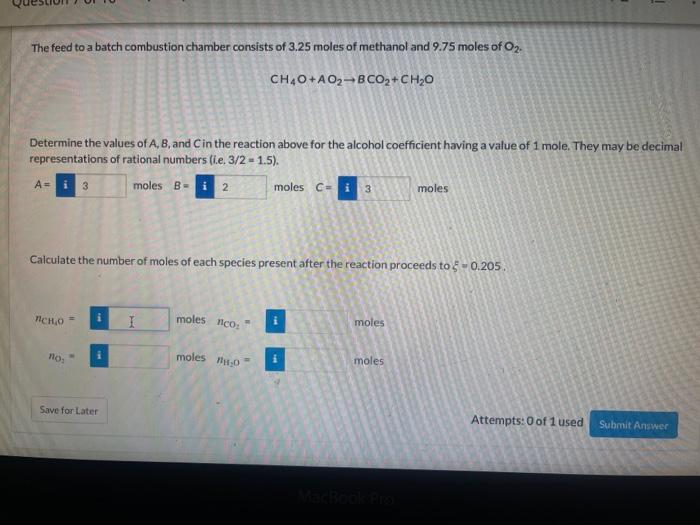
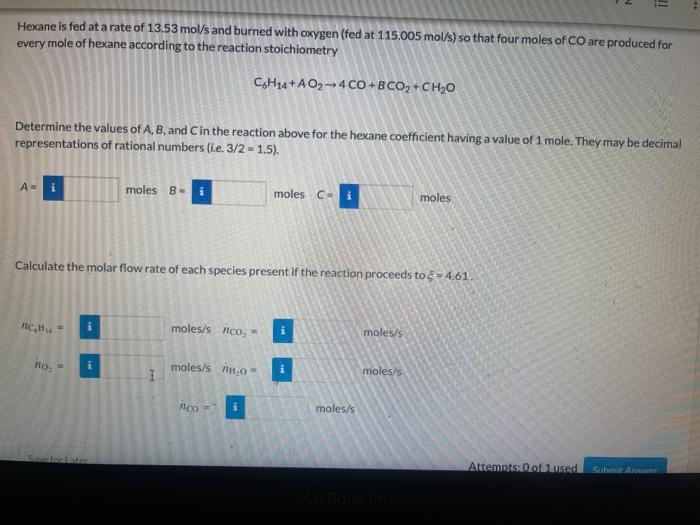
- 1 The feed to a batch combustion chamber consists of 3.25 moles of methanol and 9.75 moles of Oz. CH4O+A02BCO2+ CH20 Determine the values of A, B, and in the reaction above for the alcohol coefficient having a value of 1 mole. They may be decimal representations of rational numbers (ie. 3/2 - 1.5). moles B- i 2 moles Cu i 3 moles A= 3 Calculate the number of moles of each species present after the reaction proceeds to - 0.205. NCHO i moles ico: moles no: moles 0 moles Save for Later Attempts: 0 of 1 used Submit Answer = Hexane is fed at a rate of 13.53 mol/s and burned with oxygen (fed at 115.005 mol/s) so that four moles of CO are produced for every mole of hexane according to the reaction stoichiometry C4H14+AO2-4 CO + B CO +CHO Determine the values of A, B, and in the reaction above for the hexane coefficient having a value of 1 mole. They may be decimal representations of rational numbers (ie 3/2 - 2 -1.5). A- moles B- moles C- i moles Calculate the molar flow rate of each species present if the reaction proceeds to $ - 4.61 nc, moles/s nco. - moles/s HO i moles/s 11,0 I i moles/s /ico i moles/s Attempts of laused Siben
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


