Question: 1. The Pair for Trials 25 must be calculated because the temperatures were increased. As you warmed the flask, the air in the flask exerted
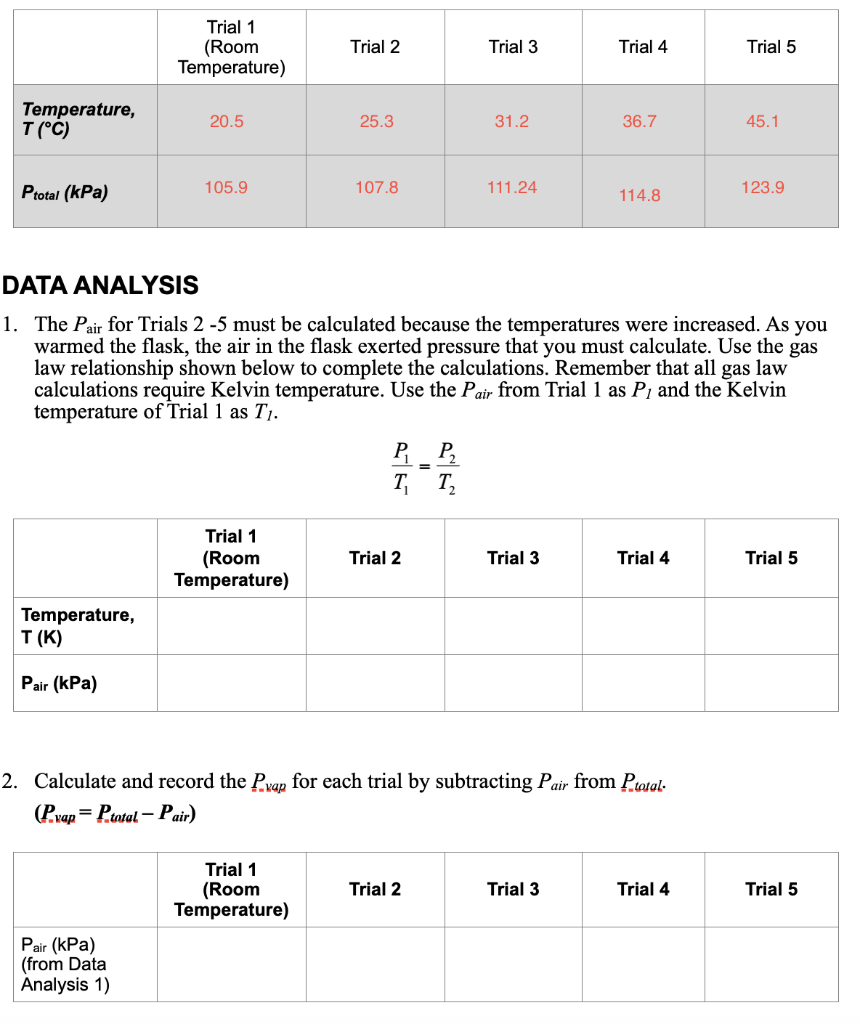
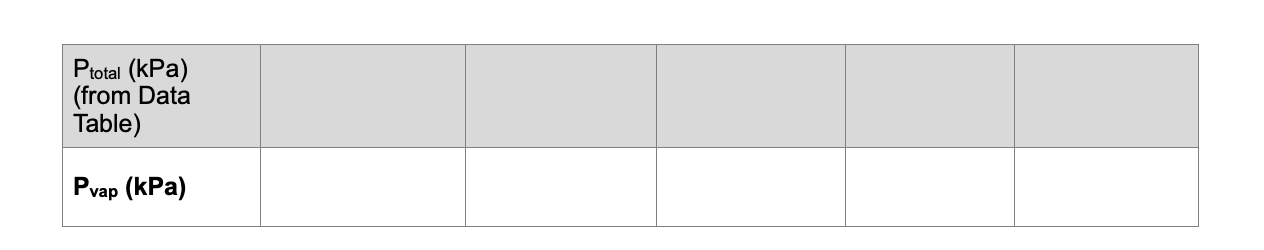
1. The Pair for Trials 25 must be calculated because the temperatures were increased. As you warmed the flask, the air in the flask exerted pressure that you must calculate. Use the gas law relationship shown below to complete the calculations. Remember that all gas law calculations require Kelvin temperature. Use the Pair from Trial 1 as P1 and the Kelvin temperature of Trial 1 as Tl. T1P1=T2P2 2. Calculate and record the Pvap for each trial by subtracting Pair from Ptotal. (Pvap=PtatalPair) 1. The Pair for Trials 25 must be calculated because the temperatures were increased. As you warmed the flask, the air in the flask exerted pressure that you must calculate. Use the gas law relationship shown below to complete the calculations. Remember that all gas law calculations require Kelvin temperature. Use the Pair from Trial 1 as P1 and the Kelvin temperature of Trial 1 as Tl. T1P1=T2P2 2. Calculate and record the Pvap for each trial by subtracting Pair from Ptotal. (Pvap=PtatalPair)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


