Question: i need help Calculations and Data Analysis 1. The pair for Trials 25 must be calculated because the temperatures were increased. As you warmed the
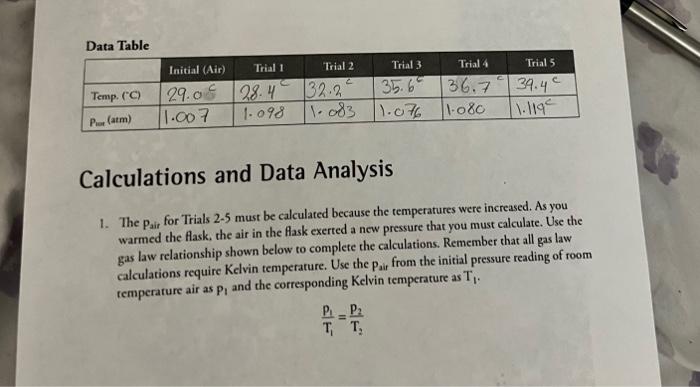

Calculations and Data Analysis 1. The pair for Trials 25 must be calculated because the temperatures were increased. As you warmed the flask, the air in the flask exerted a new pressure that you must calculate. Use the gas law relationship shown below to complete the calculations. Remember that all gas law calculations require Kelvin temperature. Use the pair from the initial pressure reading of room temperature air as P1 and the corresponding Kelvin temperature as T1. T1p1=T2p2 2. Calculate and record the psap for each trial by subtracting Pair from Prote Prop=Peot-Pair 3. Prepare an Excel Spreadsheet with the first column for the Celsius temperature, the second for the Kelvin temperature, the third column for the reciprocal Kelvin temperature, the fourth for the vapor pressure of the unknown, and the fifth for the for the natural log of the vapor pressure. Make sure this table appears copied into your lab notebook as well like it shows below. You may use Excel to calculate the Kelvin, reciprocal Kevin, and the Inpwap. but those data also need to be transferred into your laboratory notebook. Print a graph of Pvap ( y-axis) vs. Celsius temperature ( x-axis). In order to determine the heat of vaporization, Hvapy you will also need to plot the natural log of Pvap (y-axis) vs, the reciprocal of absolute temperature (x-axis). Calculate Hvap from the slope. 4. Attach the dara and calculation rables as well as the Excel Spreadsheet and the two plots to your lab report
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


