Question: 1. The rate expression below has been obtained from reactor tests for the low temperature decomposition of ozone that is catalyzed by chlorine. d[03]/dt =
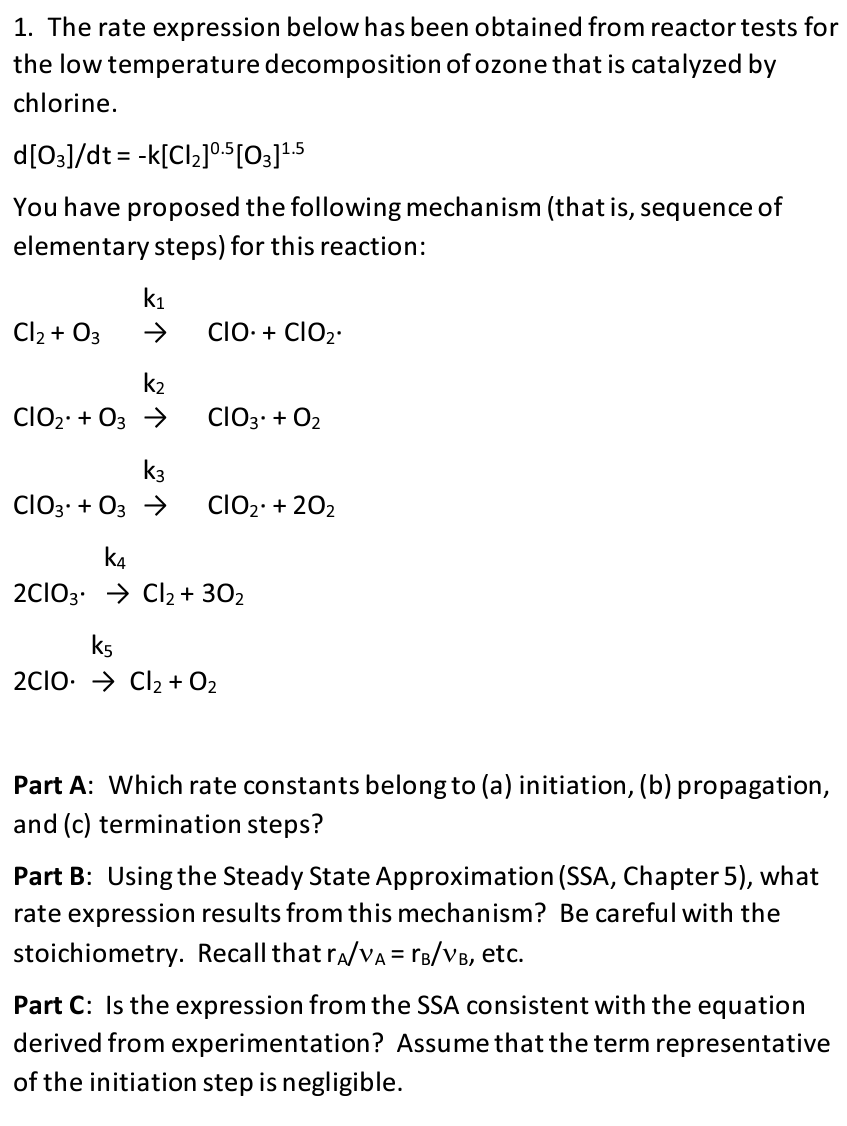
1. The rate expression below has been obtained from reactor tests for the low temperature decomposition of ozone that is catalyzed by chlorine. d[03]/dt = -k[Cl2]0.5 [O3]1.5 You have proposed the following mechanism (that is, sequence of elementary steps) for this reaction: k1 Cl2 + 03 1 CIO. + ClO2 k2 ClO2 + 03 ClO3. + O2 k3 ClOz. + 03 ClO2: +202 k4 2C103. Cl2 + 302 ks 2clo. Cl2 + O2 Part A: Which rate constants belong to (a) initiation, (b) propagation, and (c) termination steps? Part B: Using the Steady State Approximation (SSA, Chapter 5), what rate expression results from this mechanism? Be careful with the stoichiometry. Recall that ra/va= rb/VB, etc. = Part C: Is the expression from the SSA consistent with the equation derived from experimentation? Assume that the term representative of the initiation step is negligible
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


