Question: 1. The table below shows typical results from the analysis of a mineral water. In answering parts a-c, you may find the equation sheet has
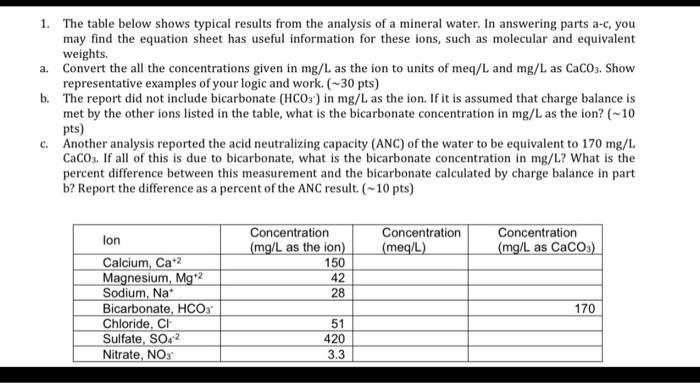
1. The table below shows typical results from the analysis of a mineral water. In answering parts a-c, you may find the equation sheet has useful information for these ions, such as molecular and equivalent weights. a. Convert the all the concentrations given in mg/L as the ion to units of meq/L and mg/L as CaCO3. Show representative examples of your logic and work. (-30 pts) b. The report did not include bicarbonate (HCO3-) in mg/L as the ion. If it is assumed that charge balance is met by the other ions listed in the table, what is the bicarbonate concentration in mg/L as the ion? (-10 pts) c. Another analysis reported the acid neutralizing capacity (ANC) of the water to be equivalent to 170 mg/L CaCO3. If all of this is due to bicarbonate, what is the bicarbonate concentration in mg/L? What is the percent difference between this measurement and the bicarbonate calculated by charge balance in part b? Report the difference as a percent of the ANC result. (-10 pts) Concentration (meq/L) Concentration (mg/L as CaCO3) Concentration (mg/L as the ion) 150 42 28 lon Calcium, Cat2 Magnesium, Mg 2 Sodium, Na Bicarbonate, HCO3 Chloride, CI Sulfate, SO42 Nitrate, NOS 170 51 420 3.3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


