Question: 1) Use the solubility table and classify each compound as water soluble or water insoluble Li3PO4Ba(OH)2SrSO4Ni(NO3)2 Solubility Table Water-Soluble Compounds Insoluble Exceptions Compounds containing an
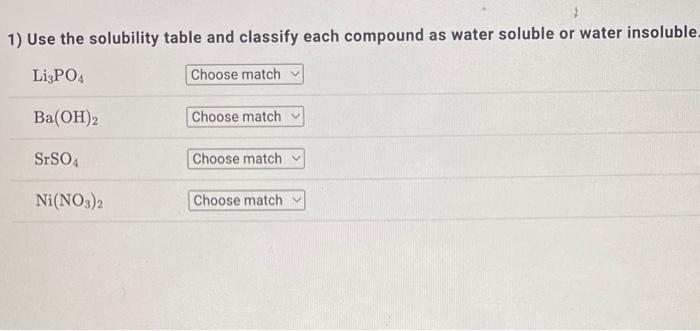
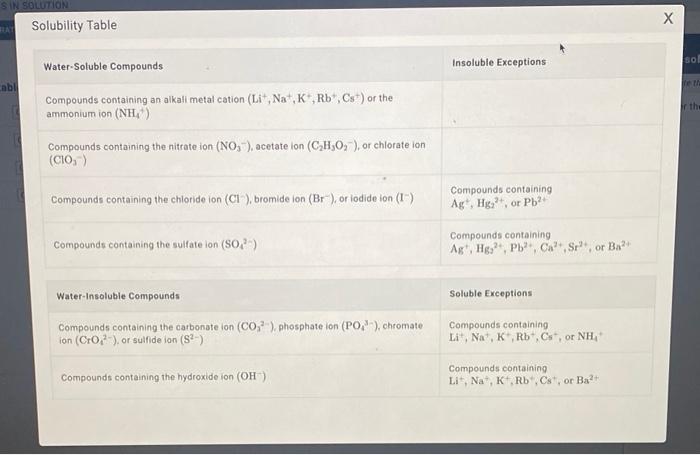
1) Use the solubility table and classify each compound as water soluble or water insoluble Li3PO4Ba(OH)2SrSO4Ni(NO3)2 Solubility Table Water-Soluble Compounds Insoluble Exceptions Compounds containing an alkall metal cation (Li+,Na+,K+,Rb+,Cs+)or the ammonium ion (NH4) Compounds containing the nitrate ion (NO3), acetate ion (C2H3O2), or chlorate ion (ClO3) Compounds containing the chloride ion (Cl), bromide ion (Br), or iodide ion (I) Compounds containing Ag+,Hg22+, or Pb2+ Compounds containing the sulfate ion (SO42) Compounds containing Ag+,Hg22+,Pb2+,Ca2+,Sr2+, or Ba2+ Water-Insoluble Compounds Soluble Exceptions Compounds containing the carbonate ion (CO32), phosphate ion (PO43), chromate Compounds containing ion (CrO42). or sulfide ion (S2) Li+,Na+,K+,Rb+,Cs+, or NH4+ Compounds containing the hydroxide ion (OH) Compounds containing Li+,Na+,K+,Rb+,Cs+, or Ba2+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


