Question: Consider the following molecules: a. CH4 b. CH3Cl c. BeF2 d. CO2 a. In which compound are the bonds most polar? b. Which compounds are
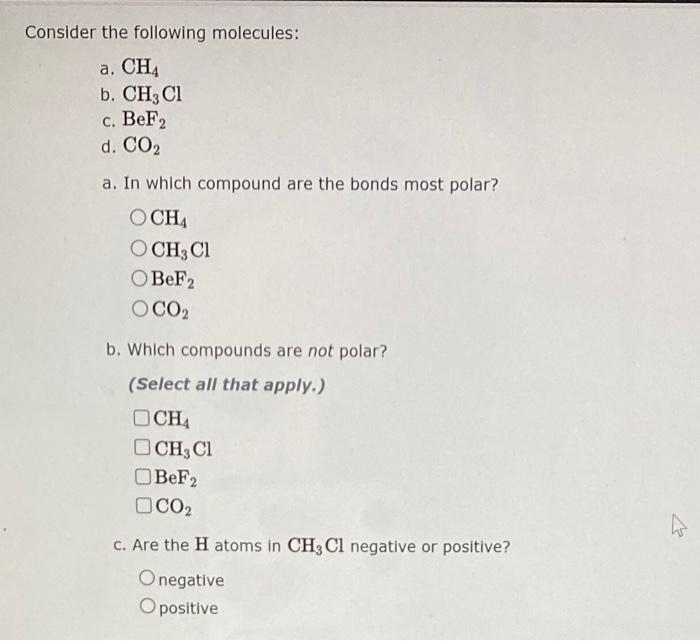
Consider the following molecules: a. CH4 b. CH3Cl c. BeF2 d. CO2 a. In which compound are the bonds most polar? b. Which compounds are not polar? (Select all that apply.) CH4CH3ClBeF2CO2 c. Are the H atoms in CH3Cl negative or positive? negative positive
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


