Question: 1. What does equilibrium mean? Does NaCl(s)NaCl(aq) have an equilibrium? Solubility (and precipitation reactions, see summary page 2 and rules page 6 or in textbook)
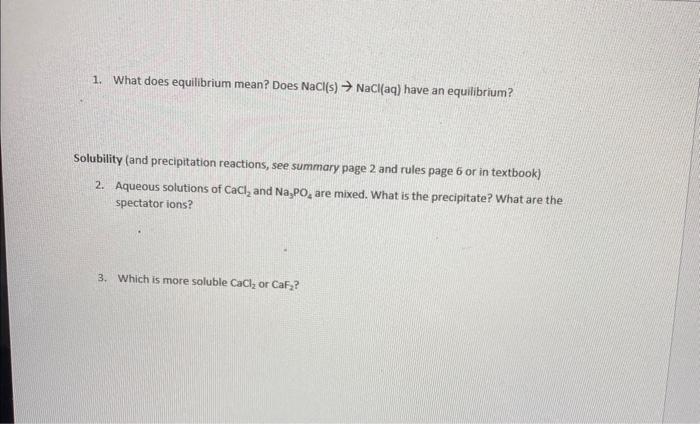
1. What does equilibrium mean? Does NaCl(s)NaCl(aq) have an equilibrium? Solubility (and precipitation reactions, see summary page 2 and rules page 6 or in textbook) 2. Aqueous solutions of CaCl2 and Na3PO4 are mixed. What is the precipitate? What are the spectator ions? 3. Which is more soluble CaCl2 or CaF2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


