Question: 1. What system is being studied? Write the reaction equation only. 2. By making reference to your observations, explain how temperature shifts the equilibrium of

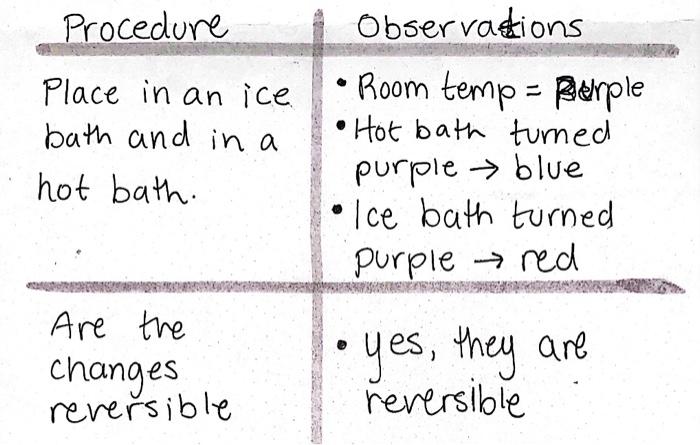
1. What system is being studied? Write the reaction equation only. 2. By making reference to your observations, explain how temperature shifts the equilibrium of the reaction: [Co(H2O)6]2+(aq)+4Cl(aq)CoCl42(aq)+6H2O Hint: Include "heat" (as a reactant or as a product) in the above reaction. Then explain using the reaction (i.e. using the longer/shorter equilibrium arrows) in both cases (when the system was heated and when the system was cooled). 3. Is the forward reaction exothermic or endothermic and explain? ProcedurePlaceinanicebathandinahotbath.ArethereversiblechangesObservations-Roomtemp=HerpleHotbathturnedpurplebluePcebathturnedpurplered-yes,theyarereversible
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


