Question: 1. When drawing Lewis structures, how many valence electrons does each of the following provide? H C. N F S Cl 2. Assign formal charge
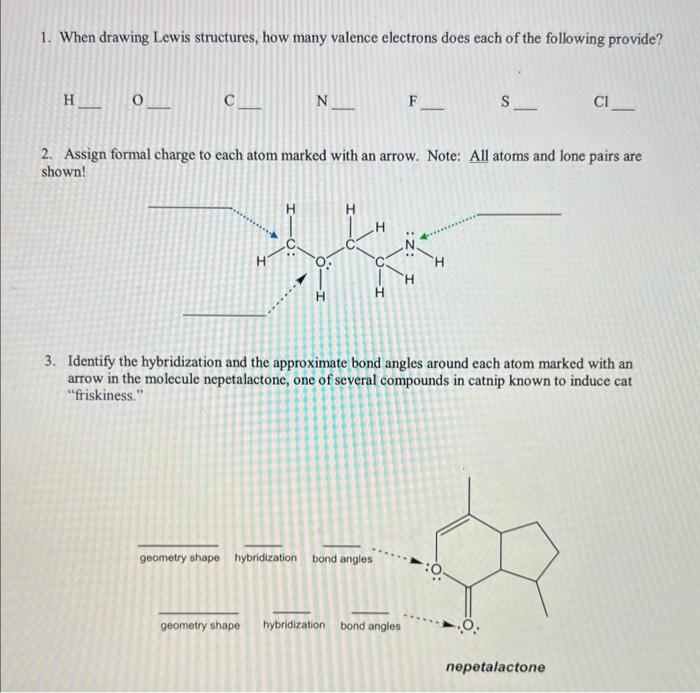
1. When drawing Lewis structures, how many valence electrons does each of the following provide? H C. N F S Cl 2. Assign formal charge to each atom marked with an arrow. Note: All atoms and lone pairs are shown! 3. Identify the hybridization and the approximate bond angles around each atom marked with an arrow in the molecule nepetalactone, one of several compounds in catnip known to induce cat "friskiness." geometry shape hybridization bond angles geometryshape hybridization bond angles
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


