Question: 1. Which statement is true for the pure compounds in the table below? (A) 1 and 3 are the same pure compound (B) 1 and
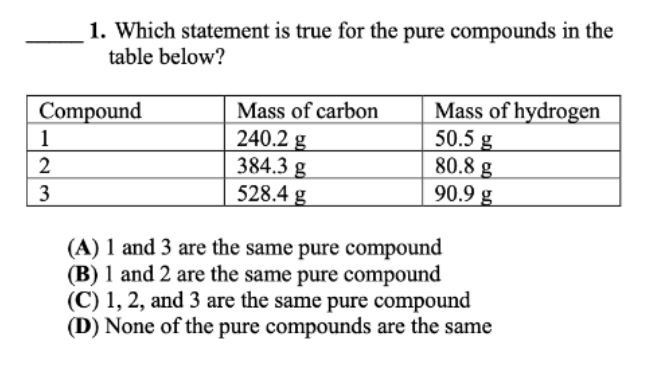
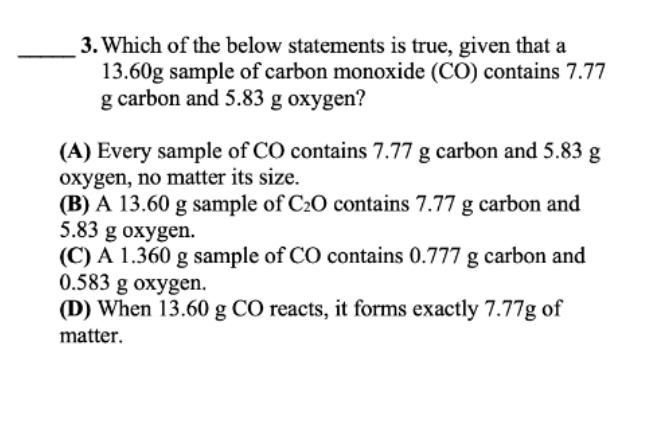
1. Which statement is true for the pure compounds in the table below? (A) 1 and 3 are the same pure compound (B) 1 and 2 are the same pure compound (C) 1, 2, and 3 are the same pure compound (D) None of the pure compounds are the same 3. Which of the below statements is true, given that a 13.60g sample of carbon monoxide (CO) contains 7.77 g carbon and 5.83g oxygen? (A) Every sample of CO contains 7.77g carbon and 5.83g oxygen, no matter its size. (B) A 13.60g sample of C2O contains 7.77g carbon and 5.83 g oxygen. (C) A 1.360g sample of CO contains 0.777g carbon and 0.583g oxygen. (D) When 13.60gCO reacts, it forms exactly 7.77g of matter
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


