Question: ) (10%) A spherical particle B is surrounded by a gas mixture. The component A diffuses through the gas mixture and reacts with B
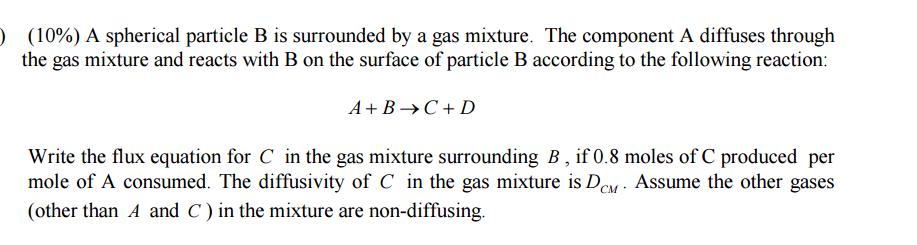
) (10%) A spherical particle B is surrounded by a gas mixture. The component A diffuses through the gas mixture and reacts with B on the surface of particle B according to the following reaction: A+B C + D Write the flux equation for C in the gas mixture surrounding B, if 0.8 moles of C produced per mole of A consumed. The diffusivity of C in the gas mixture is DCM. Assume the other gases (other than A and C) in the mixture are non-diffusing.
Step by Step Solution
3.49 Rating (159 Votes )
There are 3 Steps involved in it
87 Diffusing hom And 96 IV Ginczn Moles of c 08 ... View full answer

Get step-by-step solutions from verified subject matter experts


