Question: 10. Argon has three naturally occurring isotopes. Using the percent abundance and masses of these three isotopes, listed in the table below, please calculate the
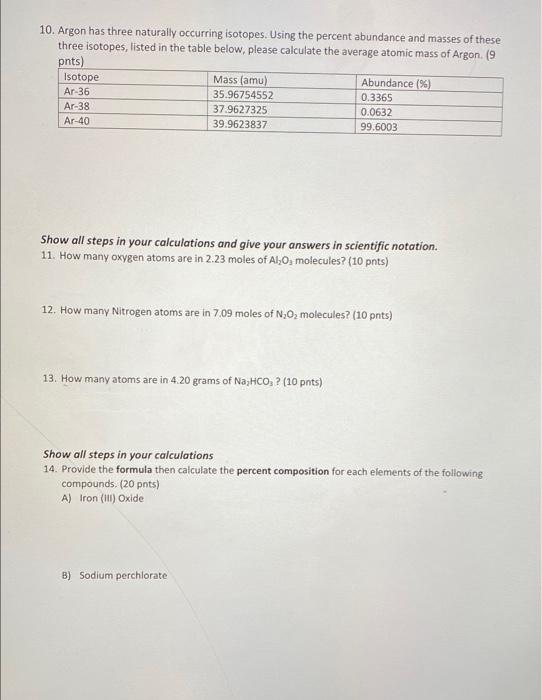


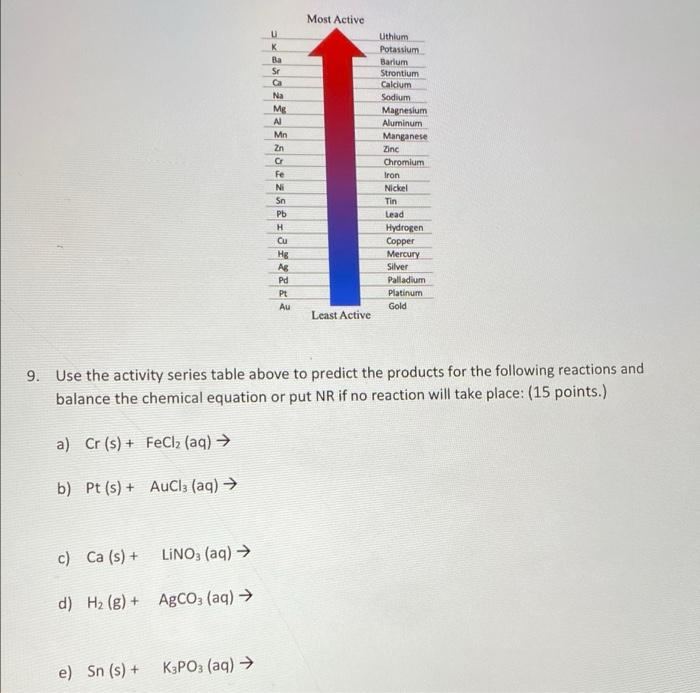
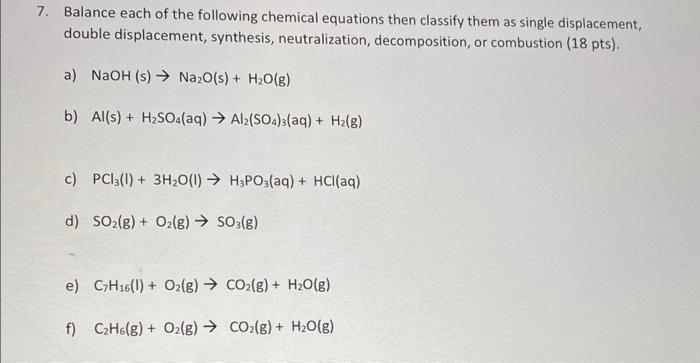
10. Argon has three naturally occurring isotopes. Using the percent abundance and masses of these three isotopes, listed in the table below, please calculate the average atomic mass of Argon (9 pnts) Isotope Mass (amu) Abundance (%) Ar-36 35.96754552 0.3365 Ar-38 37.9627325 0.0632 Ar-40 39.9623837 99.6003 Show all steps in your calculations and give your answers in scientific notation. 11. How many oxygen atoms are in 2.23 moles of Al,0, molecules? (10 pnts) 12. How many Nitrogen atoms are in 7.09 males of N,O molecules? (10 pnts) 13. How many atoms are in 4.20 grams of Na,HCO,? (10 pnts) Show all steps in your calculations 14. Provide the formula then calculate the percent composition for each elements of the following compounds. (20 pnts) A) Iron (II) Oxide B) Sodium perchlorate 15. When solid red phosphorus, Pa, is burned in air, the phosphorus combines with oxygen, producing a choking cloud of tetraphosphorus decoxide. Write the balanced chemical equation for this reaction. (10 pnts) 17. Write all equation for the reaction between Barium Bromide and Copper (II) Acetate, please include the states of the reactants and products for full credit (15 pnts) balanced chemical equation Complete ionic equation Net Ionic equation Most Active Mg Mn 3x3x02322 23232222 Uthlum Potassium Barium Strontium Calcium Sodium Magnesium Aluminum Manganese Zinc Chromium Iron Nickel Tin Lead Hydrogen Copper Mercury Silver Palladium Platinum Gold LLL Least Active 9. Use the activity series table above to predict the products for the following reactions and balance the chemical equation or put NR if no reaction will take place: (15 points.) a) Cr (s) + FeCl2 (aq) b) Pt (s) + AuCl2 (aq) c) Ca (s) + LINO; (aq) d) H2(g) + Agco (aq) e) Sn (s) + + K3PO3 (aq) 7. Balance each of the following chemical equations then classify them as single displacement, double displacement, synthesis, neutralization, decomposition, or combustion (18 pts). a) NaOH (s) Na2O(s) + H2O(g) b) Al(s) + H2SO4(aq) Al2(SO4)(aq) + H2(g) c) PC13(1) + 3H2O(l) H3PO3(aq) + HCl(aq) d) SO2(g) + O2(g) SO3(g) e) C7H16(1) + O2(g) CO2(g) + H2O(g) f) CzH6(g) + O2(g) CO2(g) + H2O(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


