Question: For a binary system consist of ortho-xylene (o-xylene) [species 1J and para-xylene (p-xylene) [species 21. this system is found to obey Raoult's law. It
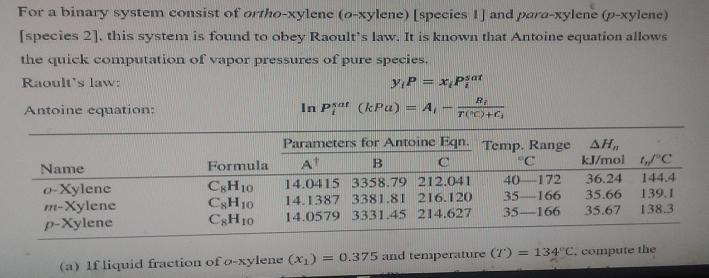

For a binary system consist of ortho-xylene (o-xylene) [species 1J and para-xylene (p-xylene) [species 21. this system is found to obey Raoult's law. It is known that Antoine equation allows the quick computation of vapor pressures of pure species. yP = x,P; Raoult's law: psat Antoine equation: In P (kPa) = A, - Parameters for Antoine Eqn. Temp. Range AH, kJ/mol "C Formula At B "C Name 172 36.24 144.4 14.0415 3358.79 212.041 14.1387 3381.81 216.120 40 o-Xylene m-Xylene p-Xylene CgH10 CSH10 CH10 139.1 138.3 35-166 35.66 35-166 35.67 14.0579 3331.45 214.627 (a) If liquid fraction of o-xylene (X1) = 0.375 and temmperature (7) = 134 C, compute the 138.3 (a) If liquid fraction of o-xylene (x1) = 0.375 and temperature (T) = 134C, compute the liquid fraction of p-xylene (x2). pressure (P), vapor fraction of o-xylene (y), vapor fraction of p-xylene (y2), vapor pressure of o-xylene(P), and vapor pressure of p- xylene (P2).- (8 marks) (b) If vapor fraction of p-xylene (y2) = 0.458 and temperature (T) = 162C, compute the vapor fraction of o-xylene (y). pressure (P), liquid fraction of o-xylene (x1), and liquid fraction of p-xylene (x2). (6 marks) %3D (c) If liquid fraction of p-xylene (x2) = 0.771 and pressure (P) = 95.27 kPa, calculate %3D the liquid fraction of o-xylene (x1) and temperature (T). (6 Marks) END OF QUESTION PAPER %24 I U Xa x2
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


