Question: (10%) Problem 6: The diagram presented represents a thermodynamic process experienced by 28.5 mmol (millimoles) of a monatomic ideal gas. The volume axis is

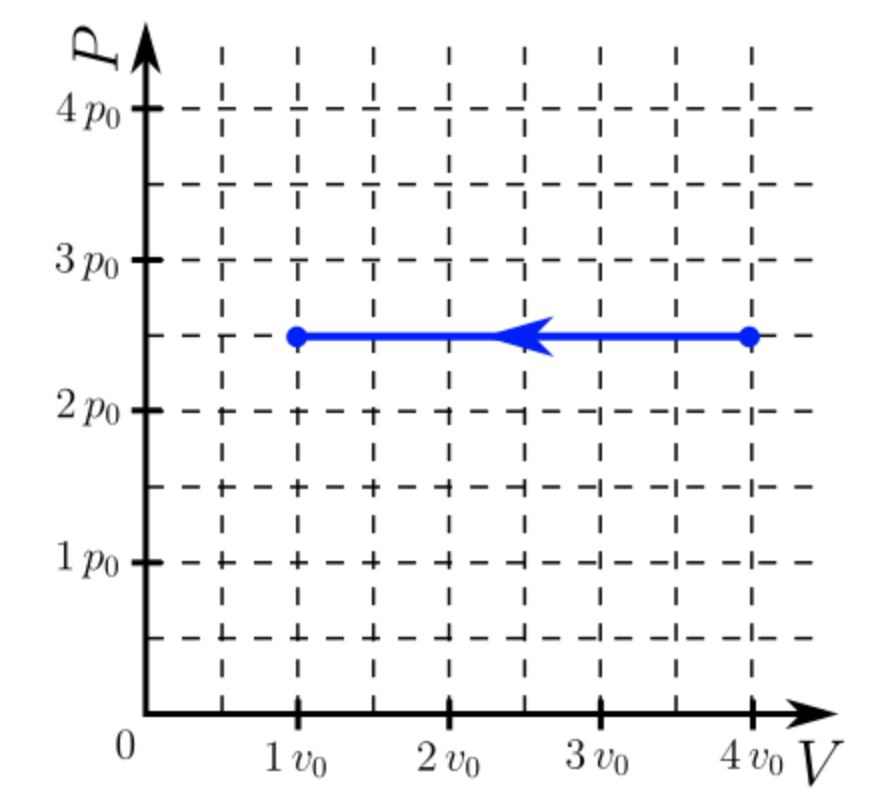
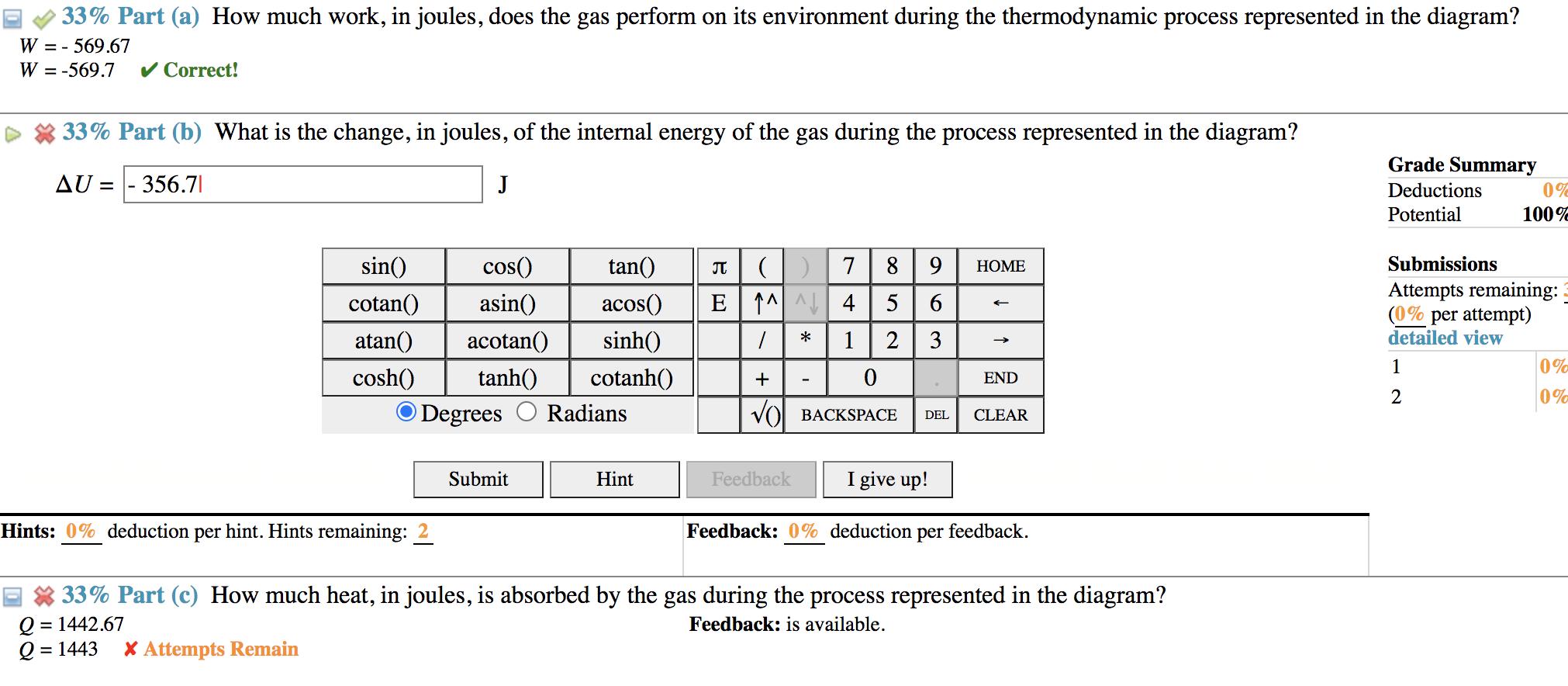
(10%) Problem 6: The diagram presented represents a thermodynamic process experienced by 28.5 mmol (millimoles) of a monatomic ideal gas. The volume axis is divided into equal increments vo = 832 cm while the pressure axis is divided into equal increments po = 0.901 atm. 4 po -|- -- | I I -|- -|- --- L + 3 po I | 2 po | 1 po - | - - + - - - - T - - - - 0 1 vo 200 - 3 vo 4 vo V 33% Part (a) How much work, in joules, does the gas perform on its environment during the thermodynamic process represented in the diagram? W = - 569.67 W = -569.7 Correct! = J 33% Part (b) What is the change, in joules, of the internal energy of the gas during the process represented in the diagram? AU 356.7| Grade Summary Deductions 0% 100% sin() cos() tan() cotan() asin() atan() acotan() sinh() acos() () 7 89 HOME E^^4 5 6 Potential Submissions Attempts remaining: (0% per attempt) * 1 2 3 3 detailed view 1 0% cosh() tanh() cotanh() Degrees Radians + 0 END 2 0% () BACKSPACE DEL CLEAR Submit Hint Feedback I give up! Hints: 0% deduction per hint. Hints remaining: Feedback: 0% deduction per feedback. 33% Part (c) How much heat, in joules, is absorbed by the gas during the process represented in the diagram? Q = 1442.67 Q = 1443 * Attempts Remain Feedback: is available.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


