Question: Problem 6 : ( 1 7 % of Assignment Value ) The diagram presented represents a thermodynamic process experienced by 3 2 . 5 mmol
Problem : of Assignment Value
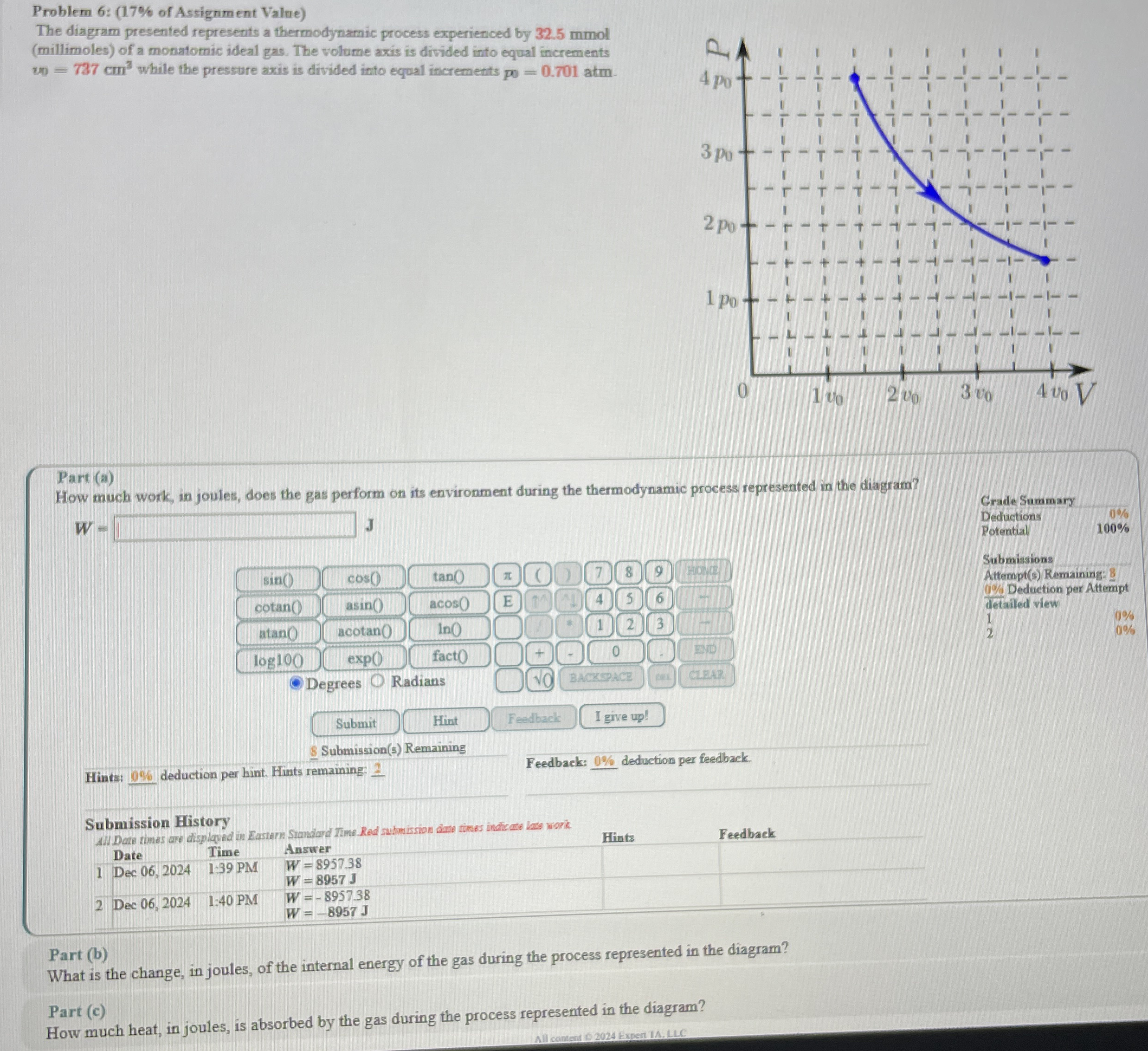
The diagram presented represents a thermodynamic process experienced by mmol millimoles of a monatomic ideal gas. The volume axis is divided into equal increments while the pressure axis is divided into equal increments atm.
Part a
How much work, in joules, does the gas perform on its environment during the thermodynamic process represented in the diagram?
Grade Summary
Deductions
Potential
Submizaions
Attempts Remaining: Deduction per Attempt detailed view
Submissions Remaining
Hints: deduction per hint. Hints remaining:
Feedback: deduction par faedback.
Submission History
Part b
What is the change, in joules, of the internal energy of the gas during the process represented in the diagram?
Part c
How much heat, in joules, is absorbed by the gas during the process represented in the diagram?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


