Question: 10. The process abc shown in the pV-diagram involves 0.0175 mole of an ideal gas. (a) What was the lowest temperature the gas reached in
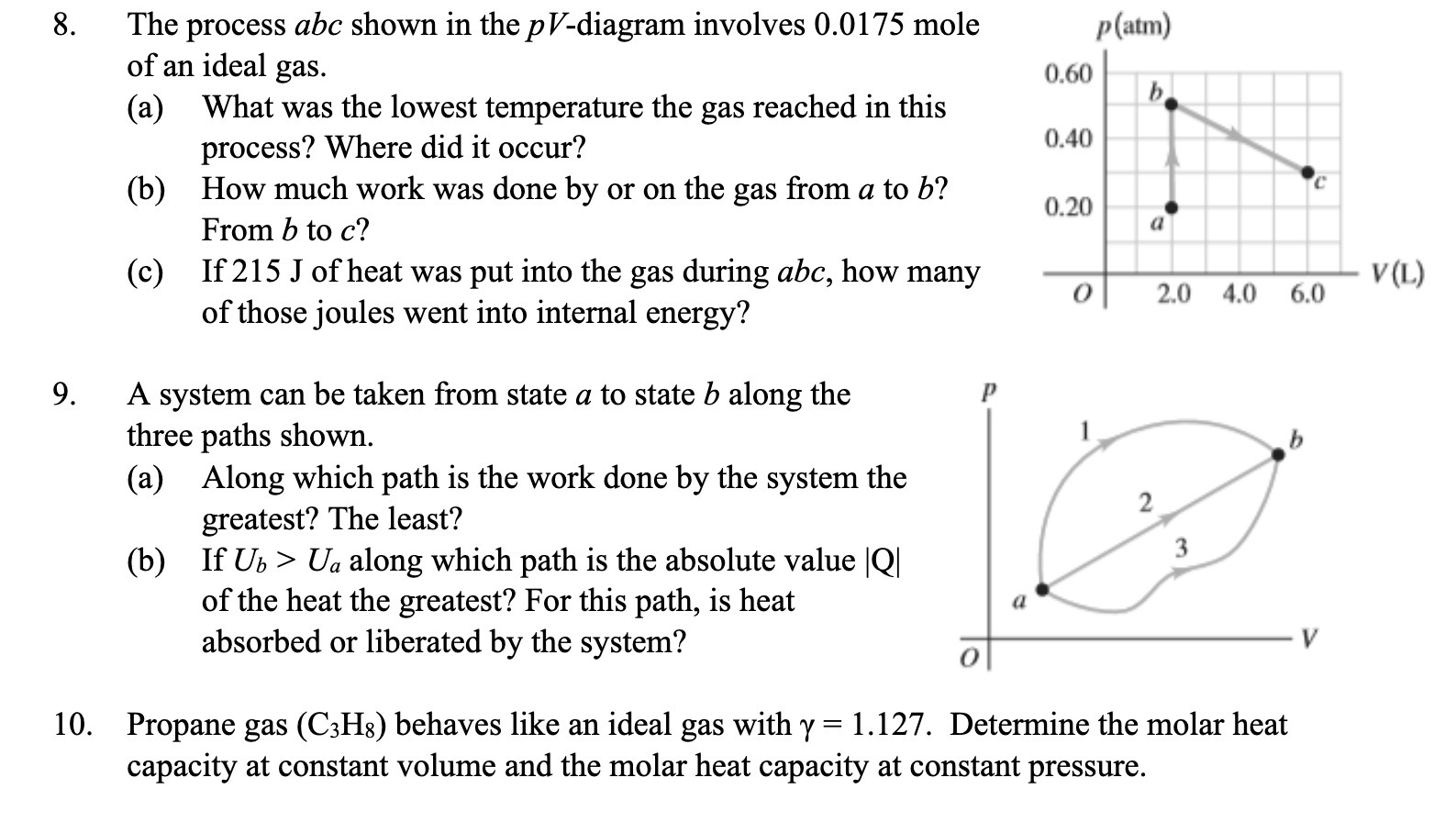
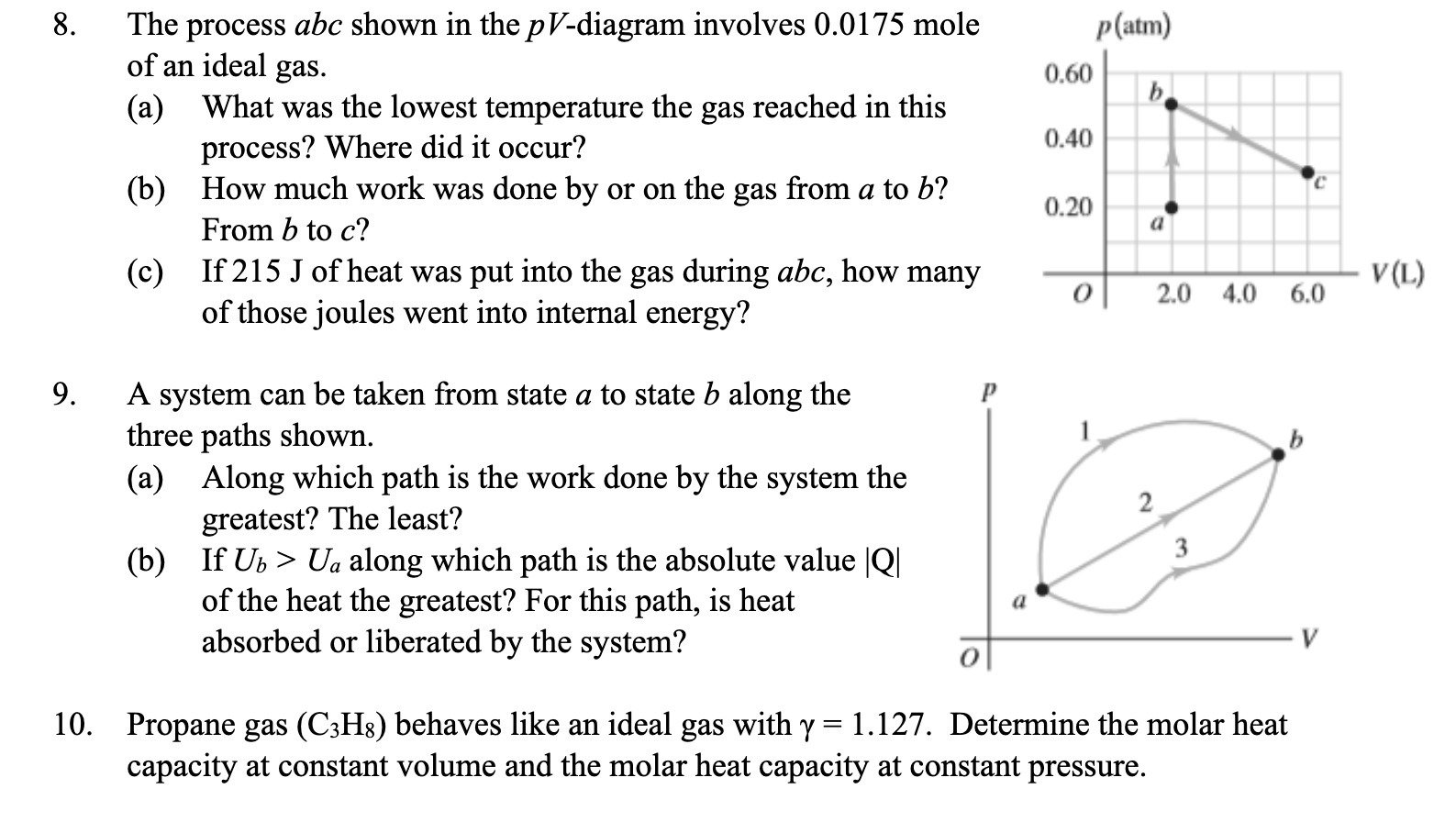
10. The process abc shown in the pV-diagram involves 0.0175 mole of an ideal gas. (a) What was the lowest temperature the gas reached in this process? Where did it occur? (b) How much work was done by or on the gas from a to [7? From I) to c? (c) If 215 J of heat was put into the gas during abc, how many of those joules went into internal energy? A system can be taken from state a to state b along the three paths shown. (a) Along which path is the work done by the system the greatest? The least? (b) If Us > Ua along which path is the absolute value |Q| of the heat the greatest? For this path, is heat absorbed or liberated by the system? Propane gas (C3Hg) behaves like an ideal gas with 7 = 1.127. Determine the molar heat capacity at constant volume and the molar heat capacity at constant pressure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


