Question: The process abc shown in the pV-diagram in Fig. E19.11 involves 0.0175 mol of an ideal gas. (a) What was the lowest temperature the
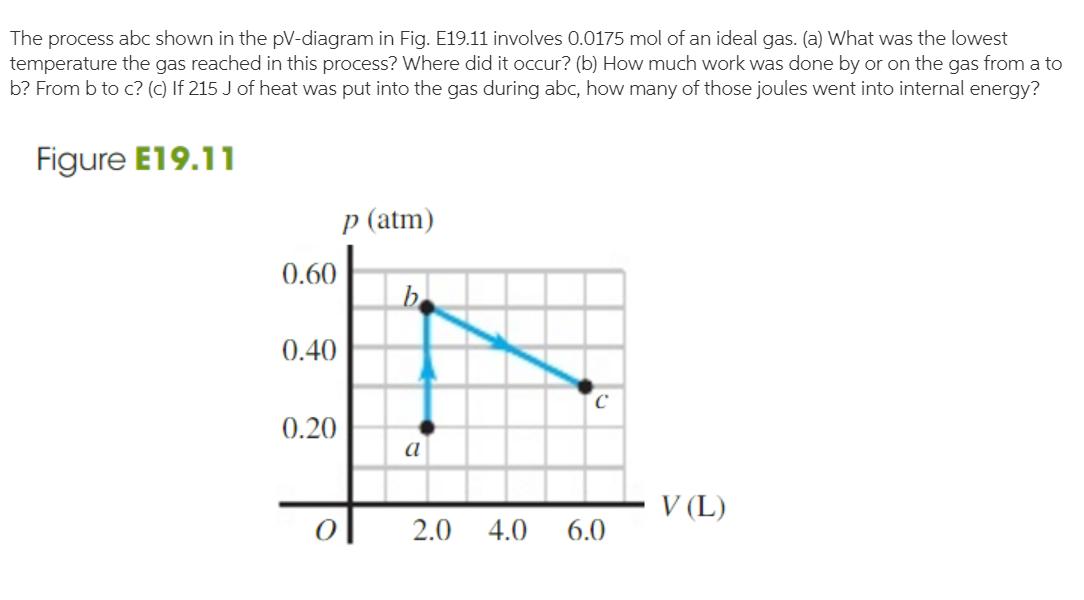
The process abc shown in the pV-diagram in Fig. E19.11 involves 0.0175 mol of an ideal gas. (a) What was the lowest temperature the gas reached in this process? Where did it occur? (b) How much work was done by or on the gas from a to b? From b to c? (c) If 215 J of heat was put into the gas during abc, how many of those joules went into internal energy? Figure E19.11 p (atm) 0.60 b 0.40 0.20 a V (L) 2.0 4.0 6.0
Step by Step Solution
3.41 Rating (148 Votes )
There are 3 Steps involved in it
Identify Part ab is isochoric but bc is not any of the familiar processes SetUp pV nRT dete... View full answer

Get step-by-step solutions from verified subject matter experts


