Question: 10.1 CaF2 and MgF2 are mutually insoluble in the solid state and form a simple binary eutectic system. Calculate the composition and temperature of the
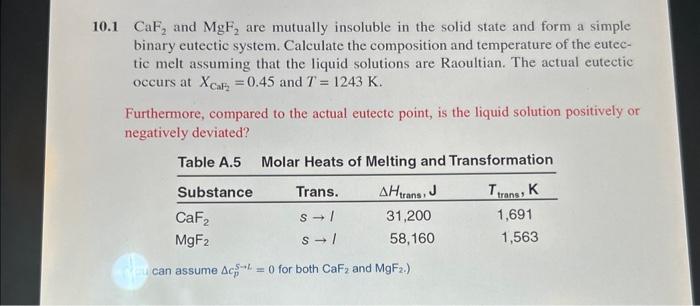
10.1 CaF2 and MgF2 are mutually insoluble in the solid state and form a simple binary eutectic system. Calculate the composition and temperature of the eutectic melt assuming that the liquid solutions are Raoultian. The actual eutectic occurs at XCaF2=0.45 and T=1243K. Furthermore, compared to the actual eutectc point, is the liquid solution positively or negatively deviated? Table A.5 Molar Heats of Meltind and Transformation can assume cp5L=0 for both CaF2 and MgF2.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


