Question: 101 chemistry 101 A solution is prepared by dissolving 25.0 g of ammonium sulfate in enough water to make 100.0 mL of stock solution. A
101
chemistry 101
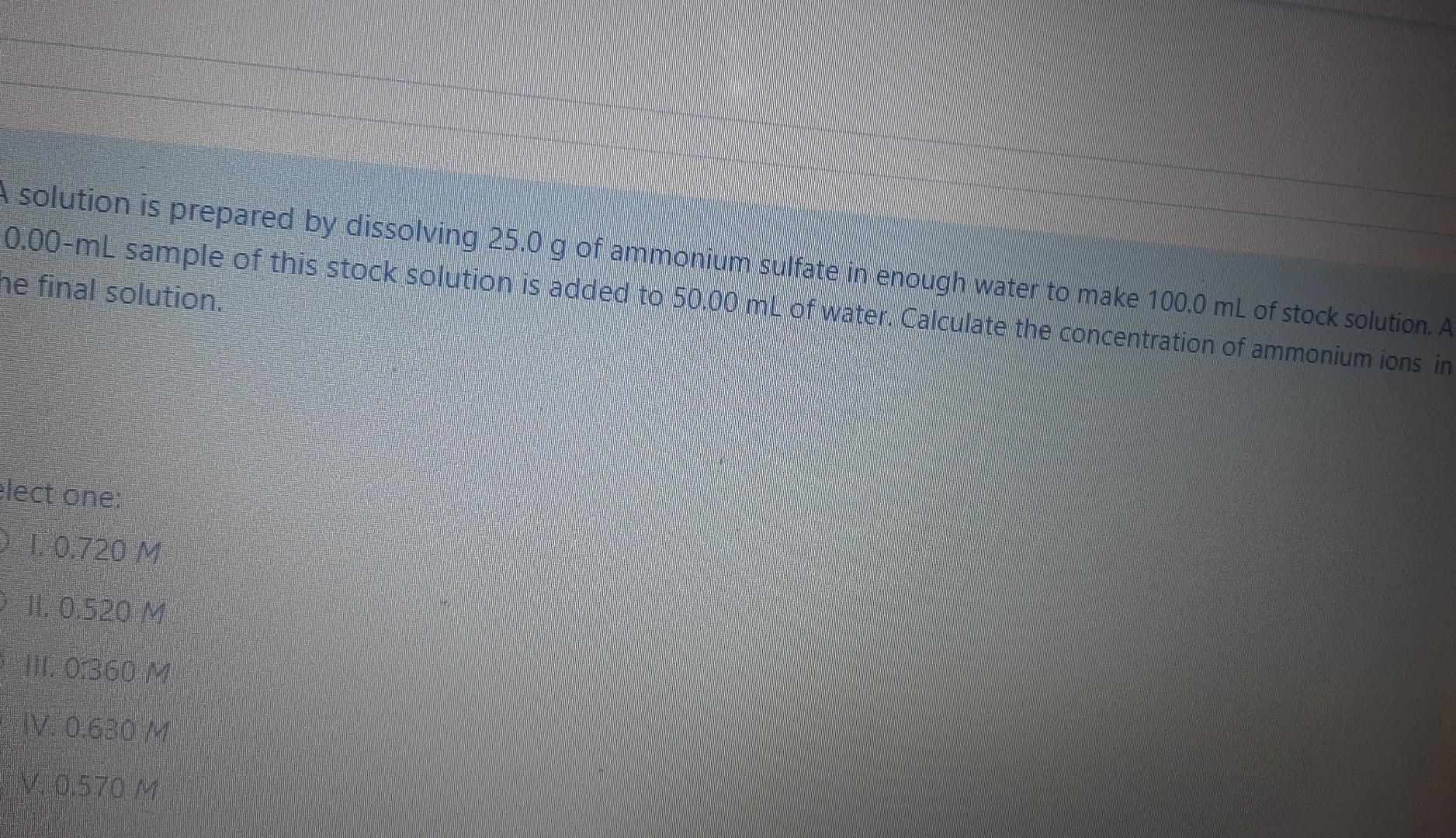
A solution is prepared by dissolving 25.0 g of ammonium sulfate in enough water to make 100.0 mL of stock solution. A 0.00-ml sample of this stock solution is added to 50,00 mL of water. Calculate the concentration of ammonium ions in he final solution. Elect one: 31.0.720 M 11. 0.520 M HD 0:360 M IV 0:630 M 0.570 M
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


