Soluble nontoxic salts such as Fe 2 (SO 4 ) 3 are often used during water purification
Question:
Soluble nontoxic salts such as Fe2(SO4)3 are often used during water purification to remove soluble toxic contaminants, because they form gelatinous hydroxides that encapsulate the contaminants and allow them to be filtered from the water.
(a) Calculate the molar solubility in water of Fe(OH)3 at 25°C.
(b) What is the concentration of hydroxide ions in a saturated solution of Fe(OH)3? Calculate the pH of the solution.
(c) Discuss whether your result in part (b) is reasonable for a solution of a basic hydroxide. Explain what assumptions were made in your calculations and evaluate the validity of the assumptions.
(d) A simplified equation for the reaction of Fe3+ ions with water is Fe3+(aq) + 6 H2O(l) ⇌ Fe(OH)3(s) + 3 H3O+(aq). Use the data in Table 6I.1 and Kw to calculate the equilibrium constant for this reaction.
(e) If 10.0 g of Fe2(SO4)3 is dissolved in enough water to make up 1.00 L of aqueous solution and the pH of the solution is raised to 8.00 by addition of NaOH, what mass of solid Fe(OH)3 will form?
(f) To test for the ability of Fe2(SO4)3 to remove chloride ions from water, a standard aqueous solution containing 24.72 g of NaCl in 1.000 L of solution was prepared. A sample of the NaCl solution of volume 25.00 mL was then combined with the mixture described in part (e) and stirred. The Fe(OH)3 precipitate containing encapsulated chloride ion was removed by filtration, then dissolved in acid. An aqueous solution of AgNO3 was then added to the resulting solution and the solid AgCl formed filtered and dried. The mass of AgCl was 0.604 g. What percentage of the chloride ion in the sample had been removed from the solution?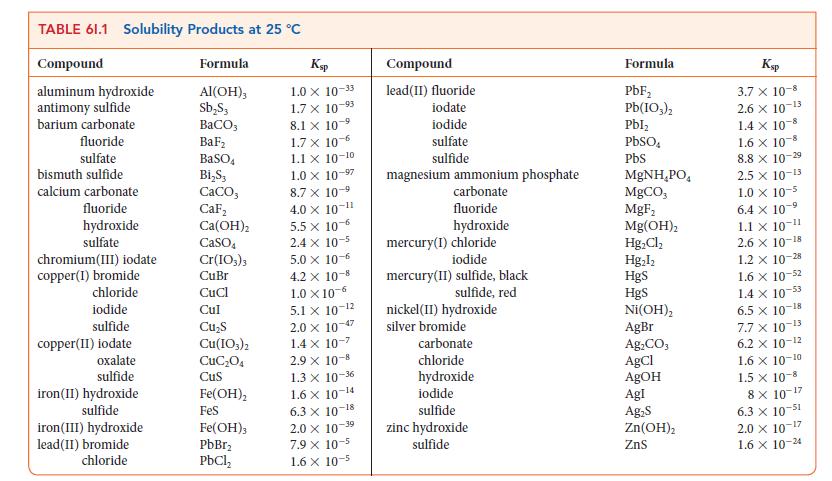
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





