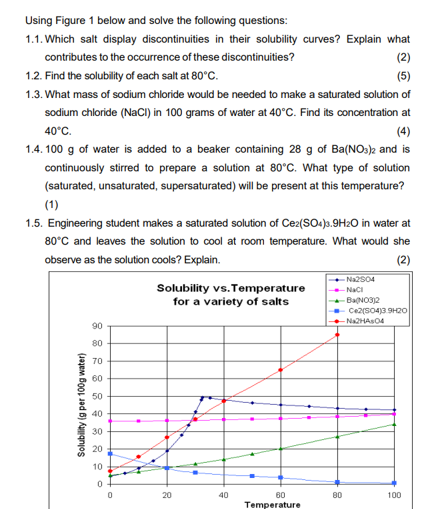
Question: 1.1 and 1.2 please Using Figure 1 below and solve the following questions: 1.1. Which salt display discontinuities in their solubility curves? Explain what contributes
1.1 and 1.2 please
Using Figure 1 below and solve the following questions: 1.1. Which salt display discontinuities in their solubility curves? Explain what contributes to the occurrence of these discontinuities? (2) 1.2. Find the solubility of each salt at 80C. (5) 1.3. What mass of sodium chloride would be needed to make a saturated solution of sodium chloride (NaCl) in 100 grams of water at 40C. Find its concentration at 40C (4) 1.4. 100 g of water is added to a beaker containing 28 g of Ba(NO3)2 and is continuously stirred to prepare a solution at 80C. What type of solution (saturated, unsaturated, supersaturated) will be present at this temperature? (1) 1.5. Engineering student makes a saturated solution of Ce2(SO4)3.9H20 in water at 80C and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain. (2) Solubility vs. Temperature for a variety of salts Na2804 - NICI -Ba(NO3)2 Ce2(SO4)39H20 NAHASO4 90 80 70 60 50 Solubility to per 100g water) 40 30 20 10 0 0 20 40 80 100 80 Temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


