Question: QUESTION 1 Using Figure 1 below and solve the following questions: 1.1. Which salt display discontinuities in their solubility curves? Explain what contributes to the
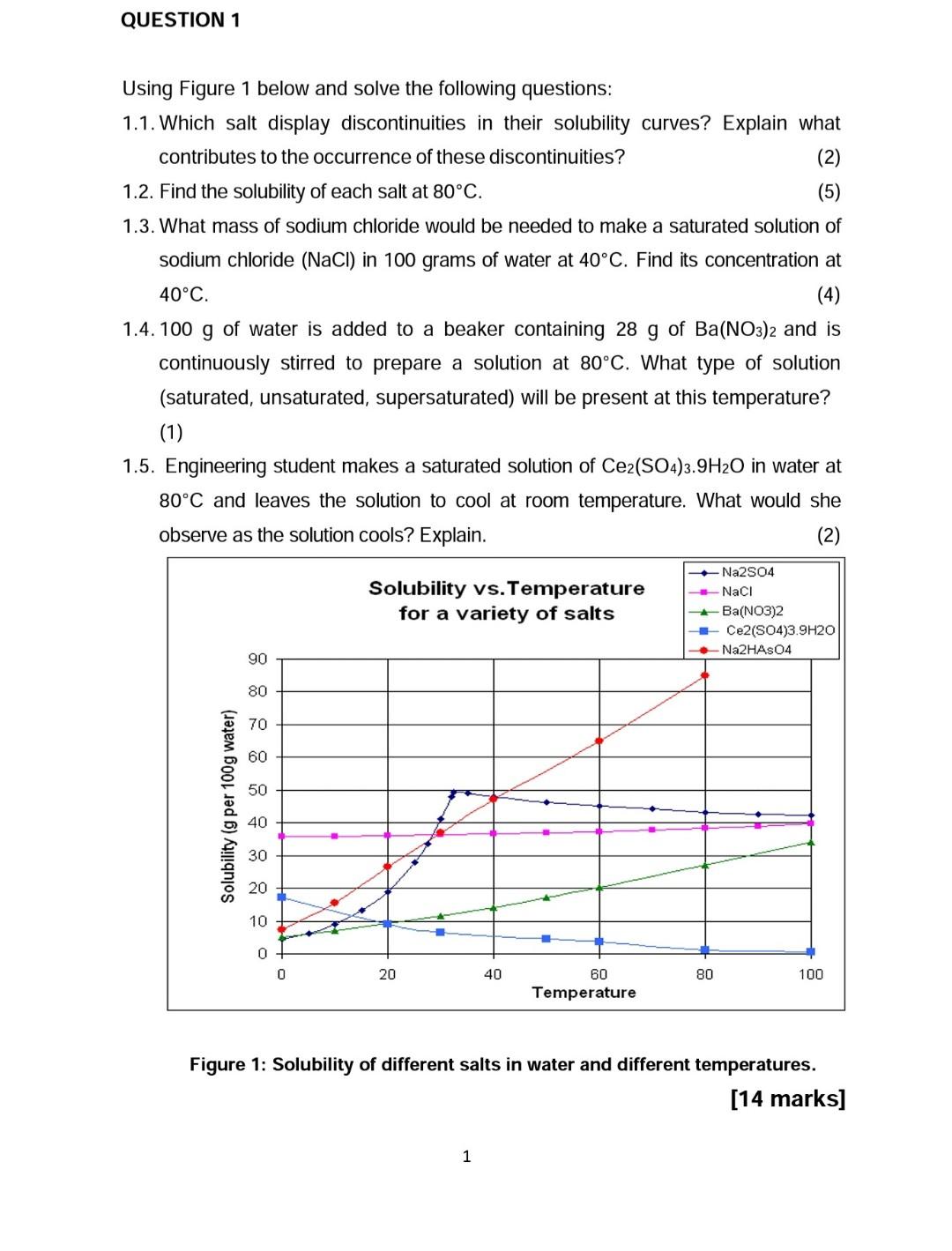
QUESTION 1 Using Figure 1 below and solve the following questions: 1.1. Which salt display discontinuities in their solubility curves? Explain what contributes to the occurrence of these discontinuities? (2) 1.2. Find the solubility of each salt at 80C. (5) 1.3. What mass of sodium chloride would be needed to make a saturated solution of sodium chloride (NaCl) in 100 grams of water at 40C. Find its concentration at 40C (4) 1.4. 100 g of water is added to a beaker containing 28 g of Ba(NO3)2 and is continuously stirred to prepare a solution at 80C. What type of solution (saturated, unsaturated, supersaturated) will be present at this temperature? (1) 1.5. Engineering student makes a saturated solution of Ce2(SO4)3.9H2O in water at 80C and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain. (2) Solubility vs. Temperature for a variety of salts Na2SO4 Naci Ba(NO3)2 Ce2(SO4)3.9H20 Na2HAS04 90 80 70 60 50 Solubility (g per 100g water) 40 30 20 10 0 0 20 40 80 100 60 Temperature Figure 1: Solubility of different salts in water and different temperatures. [14 marks] 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


