Question: 11). Identify the oxidizing agent and reducing agent in each of the following: a). 2 Al(s) + 3 H2SO4 (aq) Al2(SO4)3 (aq) + 3 H2
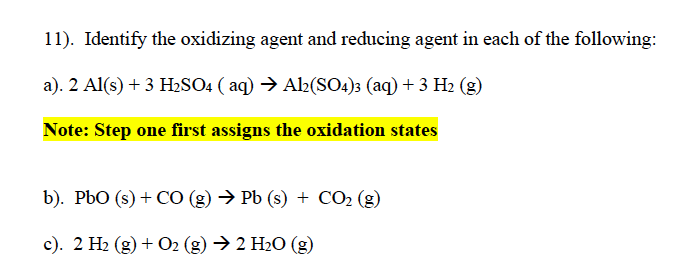
11). Identify the oxidizing agent and reducing agent in each of the following: a). 2 Al(s) + 3 H2SO4 (aq) Al2(SO4)3 (aq) + 3 H2 (9) Note: Step one first assigns the oxidation states b). PbO (s) + CO (g) Pb (s) + CO2(g) c). 2 H2 (g) + O2(g) 2 H20 (g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


