Question: 1.(10 points) Using the data in the table, determine the rate law, including rate constant, for the following general reaction A+B+CD a. What is the
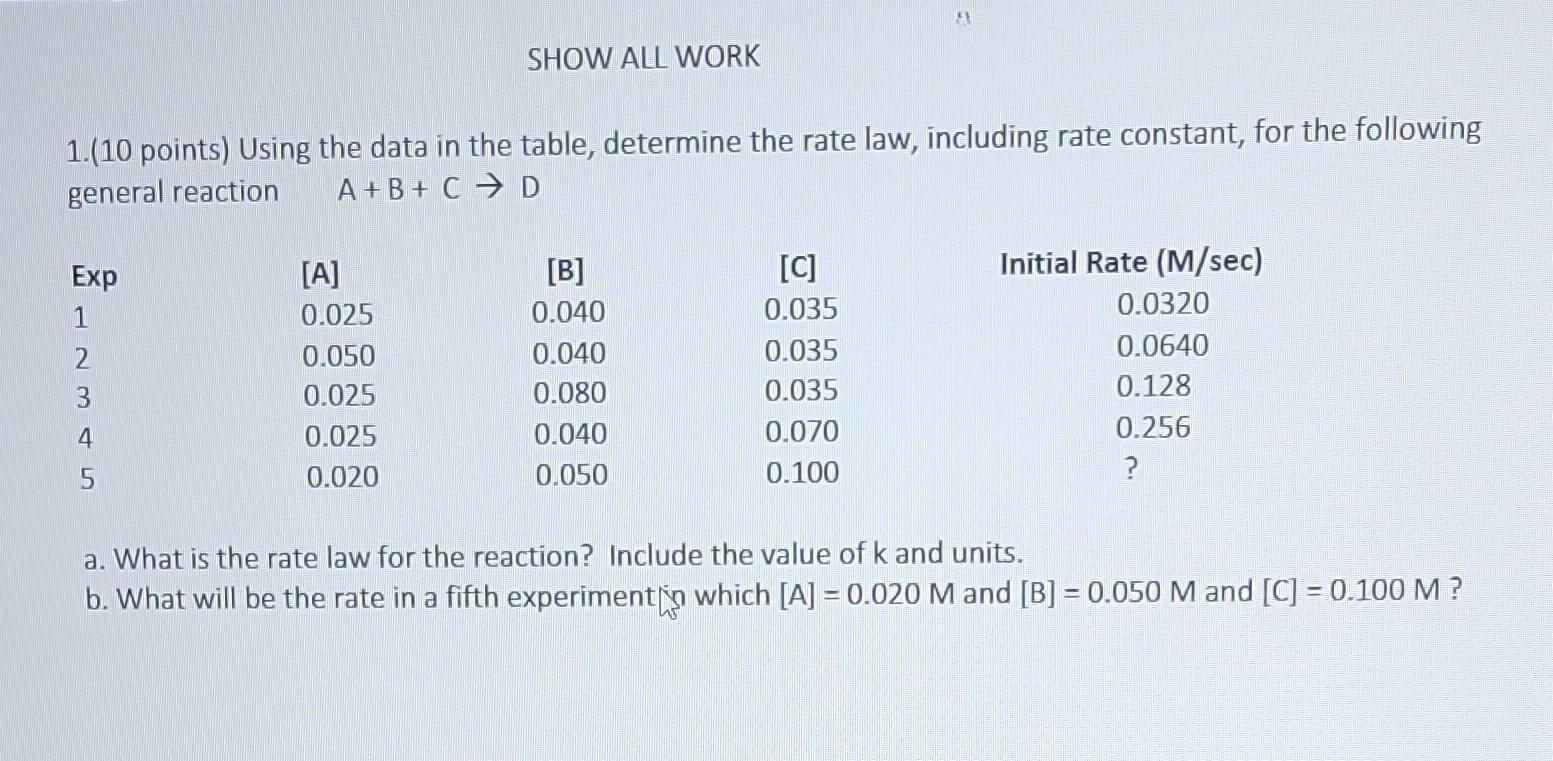
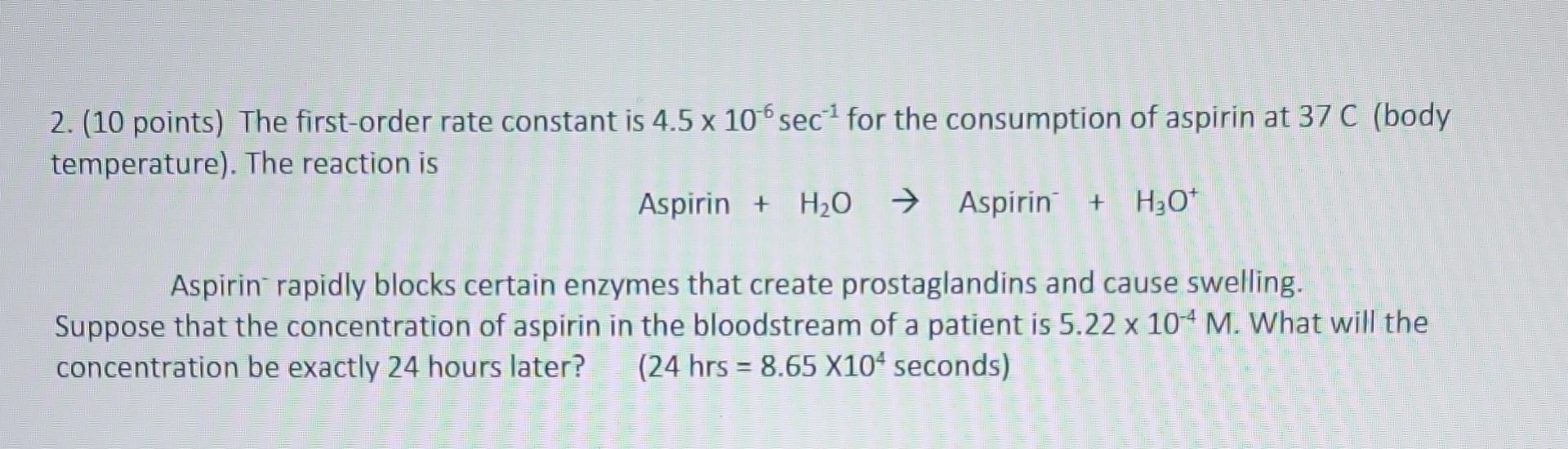
1.(10 points) Using the data in the table, determine the rate law, including rate constant, for the following general reaction A+B+CD a. What is the rate law for the reaction? Include the value of k and units. b. What will be the rate in a fifth experiment [ n which [A]=0.020M and [B]=0.050M and [C]=0.100M ? 2. (10 points) The first-order rate constant is 4.5106sec1 for the consumption of aspirin at 37C (body temperature). The reaction is Aspirin+H2OAspirin+H3O+ Aspirin rapidly blocks certain enzymes that create prostaglandins and cause swelling. Suppose that the concentration of aspirin in the bloodstream of a patient is 5.22104M. What will the concentration be exactly 24 hours later? ( 24hrs=8.65104 seconds)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


