Question: 12. The Is and 2 cubitals can each accommodate two electrons. How many electrons can occupy the 2p subshell in an atom! 1. Complete Table
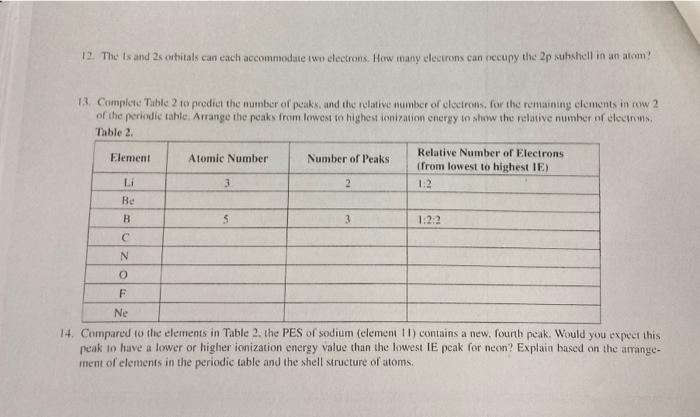
12. The Is and 2 cubitals can each accommodate two electrons. How many electrons can occupy the 2p subshell in an atom! 1. Complete Table 2 to predict the number of peaks, and the relative number of cloctrons. for the remaining elements in row 2 of the periodic table. Arrange the peaks from lowest to highest ionization energy to show the relative number of electris, Table 2. Relative Number of Electrons Element Atomic Number Number of Peaks (from lowest to highest IE) 3 2 Be B 5 3 1.2 N O F Ne 14. Compared to the elements in Table 2 the PES of sodium (element 1) contains a new. Fourth peak. Would you expect this peak to have a lower or higher ionization energy value than the lowest IE peak for neon? Explain based on the arrange- ment of elements in the periodic table and the shell structure of atoms
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


