Question: You are analyzing iodide from seawater using the cathodic stripping square-wave voltammogram technique developed by Luther et al.' The first voltammogram is below. You
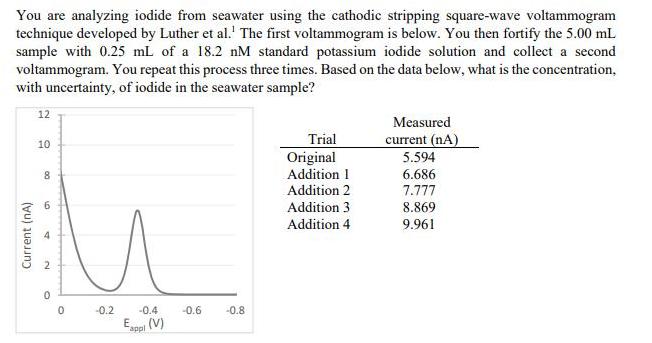
You are analyzing iodide from seawater using the cathodic stripping square-wave voltammogram technique developed by Luther et al.' The first voltammogram is below. You then fortify the 5.00 mL sample with 0.25 mL of a 18.2 nM standard potassium iodide solution and collect a second voltammogram. You repeat this process three times. Based on the data below, what is the concentration, with uncertainty, of iodide in the seawater sample? 12 Measured current (nA) 5.594 Trial 10 Original 8. Addition 1 6.686 Addition 2 7.777 6. Addition 3 8.869 Addition 4 9.961 4 -0.2 -0.4 -0.6 -0.8 Esppi (V) Current (nA) 2.
Step by Step Solution
3.37 Rating (147 Votes )
There are 3 Steps involved in it
Sample 500mL Trial Original Addition I ... View full answer

Get step-by-step solutions from verified subject matter experts


