Question: 13. Draw out the resonance structures which show how the ester interacts with the ring via resonance. Is this group an EWG or EDG? Finally,
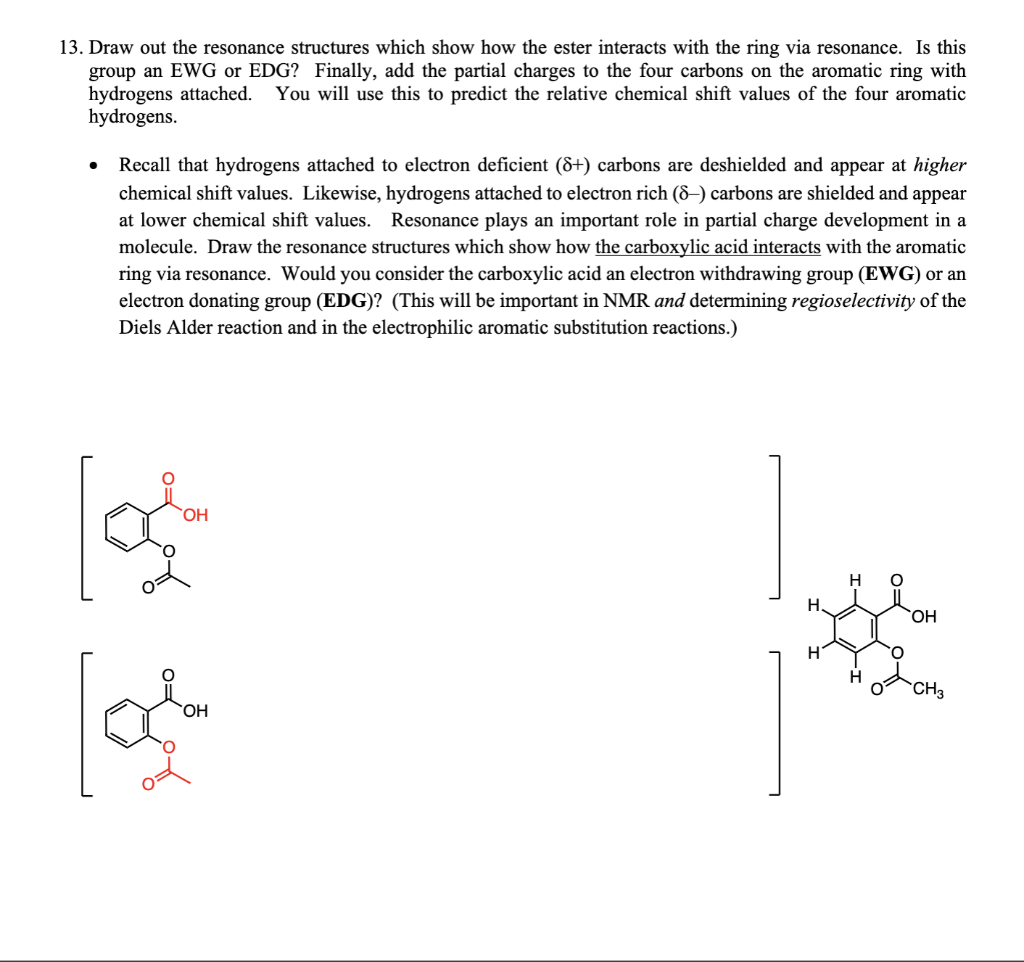
13. Draw out the resonance structures which show how the ester interacts with the ring via resonance. Is this group an EWG or EDG? Finally, add the partial charges to the four carbons on the aromatic ring with hydrogens attached. You will use this to predict the relative chemical shift values of the four aromatic hydrogens. - Recall that hydrogens attached to electron deficient (+) carbons are deshielded and appear at higher chemical shift values. Likewise, hydrogens attached to electron rich () carbons are shielded and appear at lower chemical shift values. Resonance plays an important role in partial charge development in a molecule. Draw the resonance structures which show how the carboxylic acid interacts with the aromatic ring via resonance. Would you consider the carboxylic acid an electron withdrawing group (EWG) or an electron donating group (EDG)? (This will be important in NMR and determining regioselectivity of the Diels Alder reaction and in the electrophilic aromatic substitution reactions.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


