Question: 13) What is the term for a reaction that proceeds by releasing heat energy? N2(g)+3H2(g)2NH3(g)+92KJ A) exothermic reaction B) precipitation reaction C) isothermal reaction D)
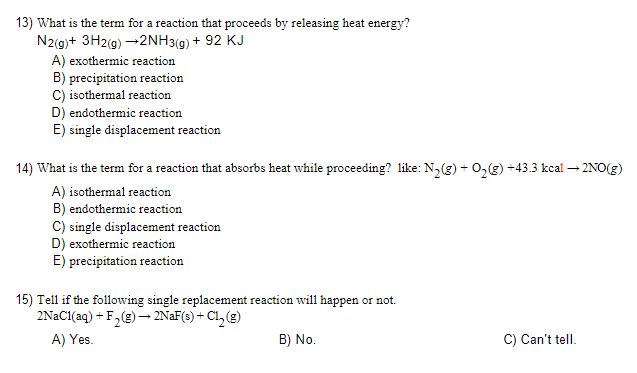
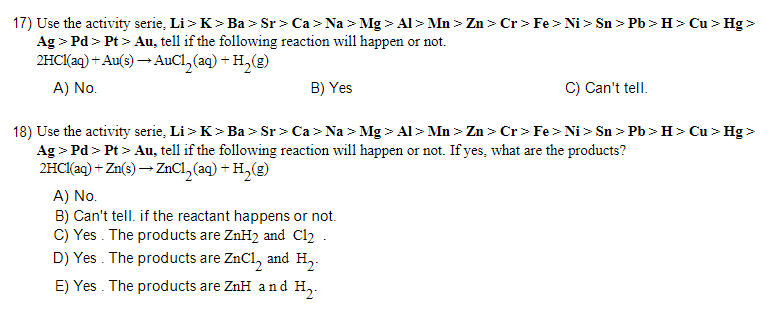
13) What is the term for a reaction that proceeds by releasing heat energy? N2(g)+3H2(g)2NH3(g)+92KJ A) exothermic reaction B) precipitation reaction C) isothermal reaction D) endothermic reaction E) single displacement reaction 14) What is the term for a reaction that absorbs heat while proceeding? like: N2(g)+O2(g)+43.3kcal2NO(g) A) isothermal reaction B) endothermic reaction C) single displacement reaction D) exothermic reaction E) precipitation reaction 15) Tell if the following single replacement reaction will happen or not. 2NaCl(aq)+F2(g)2NaF(s)+Cl2(g) A) Yes. B) No. C) Can't tell. 17) Use the activity serie, Li>K>Ba>Sr>Ca>Na>Mg>Al>Mn>Zn>Cr>Fe>Ni>Sn>Pb>H>Cu>Hg> Ag>Pd>Pt>Au, tell if the following reaction will happen or not. 2HCl(aq)+Au(s)AuCl2(aq)+H2(g) A) No. B) Yes C) Can't tell. 18) Use the activity serie, Li>K>Ba>Sr>Ca>Na>Mg>Al>Mn>Zn>Cr>Fe>Ni>Sn>Pb>H>Cu>Hg> Ag>Pd>Pt>Au, tell if the following reaction will happen or not. If yes, what are the products? 2HCl(aq)+Zn(s)ZnCl2 (aq) +H2 (g) A) No. B) Can't tell. if the reactant happens or not. C) Yes. The products are ZnH2 and Cl2. D) Yes. The products are ZnCl2 and H2. E) Yes. The products are ZnH and H2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


