Question: 14. Consider the following reaction sequence: S+Ek2k1(ES)1k4k3(ES)2k5P+E Develop a suitable rate expression for production formation [v=k5(ES)2] by using (a) the equilibrium approach, and (b) the
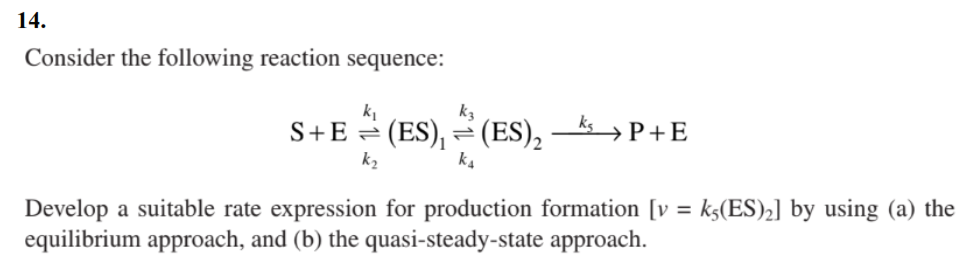
14. Consider the following reaction sequence: S+Ek2k1(ES)1k4k3(ES)2k5P+E Develop a suitable rate expression for production formation [v=k5(ES)2] by using (a) the equilibrium approach, and (b) the quasi-steady-state approach
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


